DGKδ triggers endoplasmic reticulum release of IFT88-containing vesicles destined for the assembly of primary cilia
- PMID: 28706295
- PMCID: PMC5509727
- DOI: 10.1038/s41598-017-05680-8
DGKδ triggers endoplasmic reticulum release of IFT88-containing vesicles destined for the assembly of primary cilia
Abstract
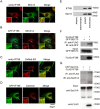
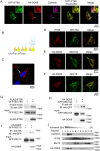
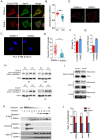
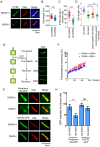
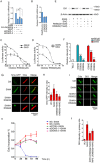
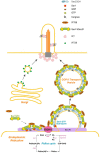
The morphogenic factor Sonic hedgehog (Shh) signals through the primary cilium, which relies on intraflagellar transport to maintain its structural integrity and function. However, the process by which protein and lipid cargos are delivered to the primary cilium from their sites of synthesis still remains poorly characterized. Here, we report that diacylglycerol kinase δ (DGKδ), a residential lipid kinase in the endoplasmic reticulum, triggers the release of IFT88-containing vesicles from the ER exit sites (ERES), thereby setting forth their movement to the primary cilium. Encoded by the gene whose mutations originally implicated the primary cilium as the venue of Shh signaling, IFT88 is known to be part of the complex B that drives the anterograde transport within cilia. We show that IFT88 interacts with DGKδ, and is associated with COPII-coated vesicles at the ERES. Using a combination of RNAi silencing and gene knockout strategies, we further show that DGKδ is required for supporting Shh signaling both in vitro and in vivo, demonstrating the physiological significance of this regulation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

