Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective
- PMID: 28706513
- PMCID: PMC5489601
- DOI: 10.3389/fmicb.2017.01205
Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective
Abstract
The continuing emergence of multi-drug resistant pathogens has sparked an interest in seeking alternative therapeutic options. Antimicrobial combinatorial therapy is one such avenue. A number of studies have been conducted, involving combinations of bacteriocins with other antimicrobials, to circumvent the development of antimicrobial resistance and/or increase antimicrobial potency. Such bacteriocin-antimicrobial combinations could have tremendous value, in terms of reducing the likelihood of resistance development due to the involvement of two distinct mechanisms of antimicrobial action. Furthermore, antimicrobial synergistic interactions may also have potential financial implications in terms of decreasing the costs of treatment by reducing the concentration of an expensive antimicrobial and utilizing it in combination with an inexpensive one. In addition, combinatorial therapies with bacteriocins can broaden antimicrobial spectra and/or result in a reduction in the concentration of an antibiotic required for effective treatments to the extent that potentially toxic or adverse side effects can be reduced or eliminated. Here, we review studies in which bacteriocins were found to be effective in combination with other antimicrobials, with a view to targeting clinical and/or food-borne pathogens. Furthermore, we discuss some of the bottlenecks which are currently hindering the development of bacteriocins as viable therapeutic options, as well as addressing the need to exercise caution when attempting to predict clinical outcomes of bacteriocin-antimicrobial combinations.
Keywords: antibiotic resistance; antimicrobials; bacteriocins; combinations; pathogens; stressors; synergy.
Figures
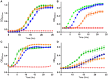
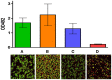
References
-
- Algburi A., Volski A., Chikindas M. L. (2015). Natural antimicrobials subtilosin and lauramide arginine ethyl ester synergize with conventional antibiotics clindamycin and metronidazole against biofilms of Gardnerella vaginalis but not against biofilms of healthy vaginal lactobacilli. Pathog. Dis. 73:ftv018 10.1093/femspd/ftv018 - DOI - PubMed
-
- Al-Seraih A., Belguesmia Y., Baah J., Szunerits S., Boukherroub R., Drider D. (2017). Enterocin B3A-B3B produced by LAB collected from infant faeces: potential utilization in the food industry for Listeria monocytogenes biofilm management. A. Van Leeuw, J. Microb. 110, 205–219. 10.1007/s10482-016-0791-5 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

