Safety of 100 µg venom immunotherapy rush protocols in children compared to adults
- PMID: 28706538
- PMCID: PMC5506672
- DOI: 10.1186/s13223-017-0204-y
Safety of 100 µg venom immunotherapy rush protocols in children compared to adults
Abstract
Background: There is a paucity of studies examining the safety of venom immunotherapy (VIT) in children. We aimed to assess the incidence of anaphylactic side effects during rush VIT in a cohort of pediatric patients and adult controls.
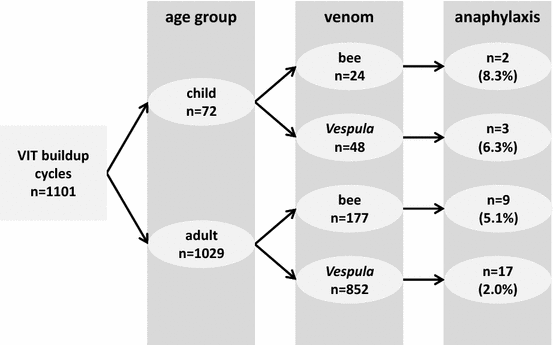
Methods: 72 consecutive cycles of VIT-buildup in 71 children/adolescents aged 7-17 years were retrospectively evaluated and compared to an adult control group (n = 981) with regard to baseline parameters (sex, causative venom, severity of index sting reaction, results of allergy testing, comorbidities) and the incidence of anaphylactic adverse reactions.
Results: Compared to adults, severe index sting-induced anaphylaxis was significantly less common in children (P = .001). Children were more likely to suffer from bee venom allergy (P < .001) and showed higher levels of bee venom-specific IgE (P = .013), but lower serum tryptase concentrations (P = .014). The overall rate of VIT-induced anaphylactic reactions was higher in children than in adults (6.9% vs 2.5%, P = .046 by univariate analysis). In the final binary logistic regression model, however, only bee VIT (P = .039; odds ratio 2.25; confidence interval 1.04-4.87) and 5-day compared to 3-day buildup protocols (P = .011; odds ratio 2.64; confidence interval 1.25-5.57) were associated with an increased risk of treatment-induced anaphylaxis. All pediatric patients finally reached and tolerated the target maintenance dose of 100 µg.
Conclusions: The higher anaphylactic reaction rate observed in pediatric patients may be attributed to a greater prevalence of bee venom allergy. VIT-induced anaphylaxis in children is usually mild and does not affect further updosing and maintenance of VIT.
Keywords: Anaphylaxis; Bee; Buildup phase; Hymenoptera; Pediatric; Risk factor; Vespula.
Figures
References
-
- Golden DB, Demain J, Freeman T, Graft D, Tankersley M, Tracy J, Blessing-Moore J, Bernstein D, Dinakar C, Greenhawt M, Khan D, Lang D, Nicklas R, Oppenheimer J, Portnoy J, Randolph C, Schuller D, Wallace D. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. doi: 10.1016/j.anai.2016.10.031. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials