Influence of cardiac nerve status on cardiovascular regulation and cardioprotection
- PMID: 28706586
- PMCID: PMC5491468
- DOI: 10.4330/wjc.v9.i6.508
Influence of cardiac nerve status on cardiovascular regulation and cardioprotection
Abstract
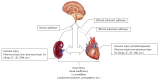
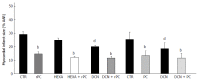
Neural elements of the intrinsic cardiac nervous system transduce sensory inputs from the heart, blood vessels and other organs to ensure adequate cardiac function on a beat-to-beat basis. This inter-organ crosstalk is critical for normal function of the heart and other organs; derangements within the nervous system hierarchy contribute to pathogenesis of organ dysfunction. The role of intact cardiac nerves in development of, as well as protection against, ischemic injury is of current interest since it may involve recruitment of intrinsic cardiac ganglia. For instance, ischemic conditioning, a novel protection strategy against organ injury, and in particular remote conditioning, is likely mediated by activation of neural pathways or by endogenous cytoprotective blood-borne substances that stimulate different signalling pathways. This discovery reinforces the concept that inter-organ communication, and maintenance thereof, is key. As such, greater understanding of mechanisms and elucidation of treatment strategies is imperative to improve clinical outcomes particularly in patients with comorbidities. For instance, autonomic imbalance between sympathetic and parasympathetic nervous system regulation can initiate cardiovascular autonomic neuropathy that compromises cardiac stability and function. Neuromodulation therapies that directly target the intrinsic cardiac nervous system or other elements of the nervous system hierarchy are currently being investigated for treatment of different maladies in animal and human studies.
Keywords: Autonomic neuropathy; Coronary blood flow regulation; Intrinsic cardiac nervous system; Ischemic conditioning; Myocardial ischemia.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest with regard to research, authorship or publication for this article.
Figures
References
-
- Kimura K, Ieda M, Fukuda K. Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ Res. 2012;110:325–336. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

