Protein ubiquitination and formation of polyubiquitin chains without ATP, E1 and E2 enzymes
- PMID: 28706640
- PMCID: PMC5485889
- DOI: 10.1039/c4sc02340d
Protein ubiquitination and formation of polyubiquitin chains without ATP, E1 and E2 enzymes
Abstract
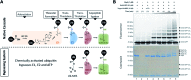
Studying protein ubiquitination is difficult due to the complexity of the E1-E2-E3 ubiquitination cascade. Here we report the discovery that C-terminal ubiquitin thioesters can undergo direct transthiolation with the catalytic cysteine of the model HECT E3 ubiquitin ligase Rsp5 to form a catalytically active Rsp5∼ubiquitin thioester (Rsp5∼Ub). The resulting Rsp5∼Ub undergoes efficient autoubiquitination, ubiquitinates protein substrates, and synthesizes polyubiquitin chains with native Ub isopeptide linkage specificity. Since the developed chemical system bypasses the need for ATP, E1 and E2 enzymes while maintaining the native HECT E3 mechanism, we named it "Bypassing System" (ByS). Importantly, ByS provides direct evidence that E2 enzymes are dispensable for K63 specific isopeptide bond formation between ubiquitin molecules by Rsp5 in vitro. Additionally, six other E3 enzymes including Nedd4-1, Nedd4-2, Itch, and Wwp1 HECT ligases, along with Parkin and HHARI RBR ligases processed Ub thioesters under ByS reaction conditions. These findings provide general mechanistic insights on protein ubiquitination, and offer new strategies for assay development to discover pharmacological modulators of E3 enzymes.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

