Induction of targeted necrosis with HER2-targeted platinum(iv) anticancer prodrugs
- PMID: 28706680
- PMCID: PMC5490001
- DOI: 10.1039/c5sc00015g
Induction of targeted necrosis with HER2-targeted platinum(iv) anticancer prodrugs
Abstract
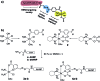
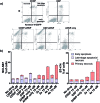
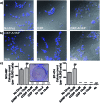
It is well-recognized that the failure of many chemotherapeutics arises due to an inability to induce apoptosis. Most cancers acquire a myriad of pro-survival adaptations, and the vast heterogeneity and accumulation of multiple often unrelated anti-apoptotic signaling pathways have been a major stumbling block towards the development of conventional chemotherapeutics, which can overcome drug resistance. We have developed highly potent and selective HER2-targeted Pt(iv) prodrugs bearing anti-HER2/neu peptides that induce targeted necrosis as a novel strategy to circumvent apoptosis-resistance. These Pt(iv)-peptide conjugates exhibit a unique biphasic mode of cytotoxicity comprising rapid killing of cancer cells via necrosis in the first phase followed by an extended and gradual phase of delayed cell death. We demonstrate that these Pt(iv)-peptide prodrugs are more potent than their Pt(ii) congeners in direct cell-killing and exhibit comparable long-term inhibition of proliferative capacity and with greater selectivity against HER2-positive cancer cells.
Figures

References
-
- Teicher B. A., in Molecular Cancer Therapeutics: Strategies for Drug Discovery and Development, John Wiley & Sons, Inc., 2005, pp. 7–40.
-
- Gerlinger M., Rowan A. J., Horswell S., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., Tarpey P., Varela I., Phillimore B., Begum S., McDonald N. Q., Butler A., Jones D., Raine K., Latimer C., Santos C. R., Nohadani M., Eklund A. C., Spencer-Dene B., Clark G., Pickering L., Stamp G., Gore M., Szallasi Z., Downward J., Futreal P. A., Swanton C. N. Engl. J. Med. 2012;366:883–892. - PMC - PubMed
-
- Galluzzi L., Vitale I., Vacchelli E., Kroemer G. Front. Oncol. 2011 doi: 10.3389/fonc.2011.00005. - DOI - PMC - PubMed
- Dinnen R. D., Drew L., Petrylak D. P., Mao Y., Cassai N., Szmulewicz J., Brandt-Rauf P., Fine R. L. J. Biol. Chem. 2007;282:26675–26686. - PubMed
- Hu X., Xuan Y. Cancer Lett. 2008;259:127–137. - PubMed
-
- Rubin I., Yarden Y. Ann. Oncol. 2001;12:S3–S8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

