Peri-exposure protection against Nipah virus disease using a single-dose recombinant vesicular stomatitis virus-based vaccine
- PMID: 28706736
- PMCID: PMC5505655
- DOI: 10.1038/npjvaccines.2016.2
Peri-exposure protection against Nipah virus disease using a single-dose recombinant vesicular stomatitis virus-based vaccine
Abstract
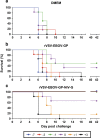
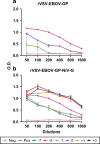
Nipah virus is a zoonotic paramyxovirus that causes severe disease in humans and animals. Due to almost yearly outbreaks in Bangladesh, and a large outbreak in Malaysia that lead to the shutdown of swine export, Nipah virus is both a threat to public health and the economy. Infection is associated with respiratory distress, encephalitis and human-to-human transmission, resulting in high case fatality rates during outbreaks. This study aims to address the amount of time needed until protection from a recombinant vesicular stomatitis virus-based vaccine candidate expressing the Nipah virus glycoprotein (G), which we have previously shown to protect hamsters and non-human primates when administered 28 days before challenge. We found that a single-dose vaccination, when administered 1 day before challenge, reduced viral load, limited pathology and fully protected hamsters from Nipah virus infection. The vaccine was even partially protective when administered at early time points following challenge with Nipah virus. These data indicate that a single administration of this vaccine to high-risk individuals, such as family members and health-care workers of infected patients, could be protective and useful for reducing human-to-human transmission and curbing an outbreak.
Conflict of interest statement
COMPETING INTERESTS The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

