Electrochemical electron beam lithography: Write, read, and erase metallic nanocrystals on demand
- PMID: 28706992
- PMCID: PMC5507638
- DOI: 10.1126/sciadv.1700234
Electrochemical electron beam lithography: Write, read, and erase metallic nanocrystals on demand
Abstract
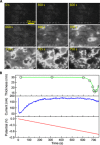
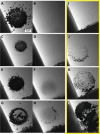
We develop a solution-based nanoscale patterning technique for site-specific deposition and dissolution of metallic nanocrystals. Nanocrystals are grown at desired locations by electron beam-induced reduction of metal ions in solution, with the ions supplied by dissolution of a nearby electrode via an applied potential. The nanocrystals can be "erased" by choice of beam conditions and regrown repeatably. We demonstrate these processes via in situ transmission electron microscopy using Au as the model material and extend to other metals. We anticipate that this approach can be used to deposit multicomponent alloys and core-shell nanostructures with nanoscale spatial and compositional resolutions for a variety of possible applications.
Figures


References
-
- I. Utke, S. Moshkalev, P. Russell, Eds., Nanofabrication Using Focused Ion and Electron Beams - Principles and Applications (Oxford Univ. Press, 2012).
-
- Mijatovic D., Eijkel J. C. T., van den Berg A., Technologies for nanofluidic systems: Top-down vs. bottom-up—A review. Lab Chip 5, 492–500 (2005). - PubMed
-
- Vieu C., Carcenac F., Pépin A., Chen Y., Mejias M., Lebib A., Manin-Ferlazzo L., Couraud L., Launois H., Electron beam lithography: Resolution limits and applications. Appl. Surf. Sci. 164, 111–117 (2000).
-
- Kim C.-S., Ahn S.-H., Jang D.-Y., Review: Developments in micro/nanoscale fabrication by focused ion beams. Vacuum 86, 1014–1035 (2012).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

