Adrenal cortical hyperplasia: diagnostic workup, subtypes, imaging features and mimics
- PMID: 28707538
- PMCID: PMC5963387
- DOI: 10.1259/bjr.20170330
Adrenal cortical hyperplasia: diagnostic workup, subtypes, imaging features and mimics
Abstract
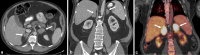
Adrenal cortical hyperplasia manifests radiologically as a non-malignant growth, or enlargement, of the adrenal glands, specifically the cortex, although the cortex cannot be definitively identified by conventional imaging. Controlled by the pituitary gland, the adrenal cortex drives critical processes, such as the production of cortisol, mineralocorticoid and sex hormones. Any disruption in the multiple enzymes and hormones involved in these pathways may cause serious or life-threatening symptoms, often associated with anatomical changes in the adrenal glands. Diagnosis and treatment of adrenal cortical hyperplasia requires a thorough clinical evaluation. As imaging has become more robust so has its role in the diagnosis and treatment of adrenal conditions. CT has been the primary modality for adrenal imaging owing to reproducibility, temporal and spatial resolution and broad access. MRI serves a complimentary role in adrenal imaging and can be used to further evaluate indeterminate CT findings or serve as an adjunct tool without the use of ionizing radiation. Ultrasound and fluoroscopy (genitography) are most commonly used in children and foetuses to evaluate congenital adrenal hyperplasia. This article will discuss the clinical presentation, laboratory workup and imaging features of adrenal cortical hyperplasia, both congenital and acquired.
Figures
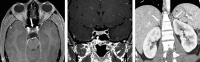
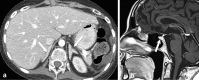
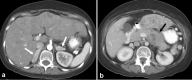
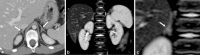
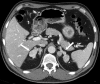
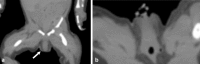
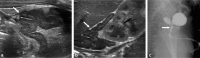
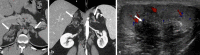
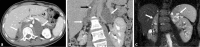
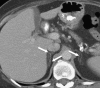
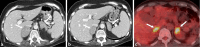
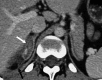
References
-
- Goldman SM, Kenney PJ. Computed Body Tomography with MRI Correlation. Vol. 1 Philadelphia, WA: Lippincot Williams & Wilkins; 2006. 1311 1311–75.
-
- Breslow MJ. Regulation of adrenal medullary and cortical blood flow. Am J Physiol 1992; 262(5 Pt 2): 1317–30. - PubMed
-
- Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, et al. American Association of Clinical endocrinologists and American Association of Endocrine Surgeons Medical guidelines for the management of Adrenal incidentalomas: executive summary of recommendations. Endocr Pract 2009; 15: 450–3. DOI: 10.4158/EP.15.5.450 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

