Biofunctionalized Hybrid Magnetic Gold Nanoparticles as Catalysts for Photothermal Ablation of Colorectal Liver Metastases
- PMID: 28707960
- PMCID: PMC5708290
- DOI: 10.1148/radiol.2017161497
Biofunctionalized Hybrid Magnetic Gold Nanoparticles as Catalysts for Photothermal Ablation of Colorectal Liver Metastases
Abstract
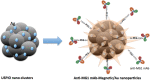
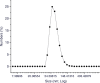
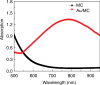
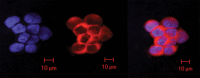
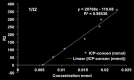
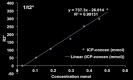
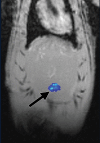
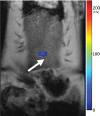
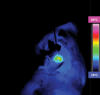
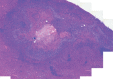
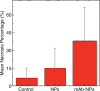
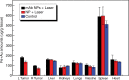
Purpose To demonstrate that anti-MG1 conjugated hybrid magnetic gold nanoparticles (HNPs) act as a catalyst during photothermal ablation (PTA) of colorectal liver metastases, and thus increase ablation zones. Materials and Methods All experiments were performed with approval of the institutional animal care and use committee. Therapeutic and diagnostic multifunctional HNPs conjugated with anti-MG1 monoclonal antibodies were synthesized, and the coupling efficiency was determined. Livers of 19 Wistar rats were implanted with 5 × 106 rat colorectal liver metastasis cell line cells. The rats were divided into three groups according to injection: anti-MG1-coupled HNPs (n = 6), HNPs only (n = 6), and cells only (control group, n = 7). Voxel-wise R2 and R2* magnetic resonance (MR) imaging measurements were obtained before, immediately after, and 24 hours after injection. PTA was then performed with a fiber-coupled near-infrared (808 nm) diode laser with laser power of 0.56 W/cm2 for 3 minutes, while temperature changes were measured. Tumors were assessed for necrosis with hematoxylin-eosin staining. Organs were analyzed with inductively coupled plasma mass spectrometry to assess biodistribution. Therapeutic efficacy and tumor necrosis area were compared by using a one-way analysis of variance with post hoc analysis for statistically significant differences. Results The coupling efficiency was 22 μg/mg (55%). Significant differences were found between preinfusion and 24-hour postinfusion measurements of both T2 (repeated measures analysis of variance, P = .025) and T2* (P < .001). Significant differences also existed for T2* measurements between the anti-MG1 HNP and HNP-only groups (P = .034). Mean temperature ± standard deviation with PTA in the anti-MG1-coated HNP, HNP, and control groups was 50.2°C ± 7.8, 51°C ± 4.4, and 39.5°C ± 2.0, respectively. Inductively coupled plasma mass spectrometry revealed significant tumor targeting and splenic sequestration. Mean percentages of tumor necrosis in the anti-MG1-coated HNP, HNP, and control groups were 38% ± 29, 14% ± 17, and 7% ± 8, respectively (P = .043). Conclusion Targeted monoclonal antibody-conjugated HNPs can serve as a catalyst for photothermal ablation of colorectal liver metastases by increasing ablation zones. © RSNA, 2017.
Figures





Comment in
-
Science to Practice: Enhancing Photothermal Ablation of Colorectal Liver Metastases with Targeted Hybrid Nanoparticles.Radiology. 2017 Dec;285(3):699-701. doi: 10.1148/radiol.2017170993. Radiology. 2017. PMID: 29155621 Free PMC article.
Similar articles
-
Science to Practice: Enhancing Photothermal Ablation of Colorectal Liver Metastases with Targeted Hybrid Nanoparticles.Radiology. 2017 Dec;285(3):699-701. doi: 10.1148/radiol.2017170993. Radiology. 2017. PMID: 29155621 Free PMC article.
-
Hepatocellular Carcinoma: Intra-arterial Delivery of Doxorubicin-loaded Hollow Gold Nanospheres for Photothermal Ablation-Chemoembolization Therapy in Rats.Radiology. 2016 Nov;281(2):427-435. doi: 10.1148/radiol.2016152510. Epub 2016 Jun 27. Radiology. 2016. PMID: 27347765 Free PMC article.
-
Photothermal ablation of pancreatic cancer cells with hybrid iron-oxide core gold-shell nanoparticles.Int J Nanomedicine. 2013;8:3437-46. doi: 10.2147/IJN.S47585. Epub 2013 Sep 6. Int J Nanomedicine. 2013. PMID: 24039426 Free PMC article.
-
Opportunities for near-infrared thermal ablation of colorectal metastases by guanylyl cyclase C-targeted gold nanoshells.Future Oncol. 2006 Dec;2(6):705-16. doi: 10.2217/14796694.2.6.705. Future Oncol. 2006. PMID: 17155897 Review.
-
[Hyperthermia on colorectal cancer: gold nanoshells-mediated photothermal therapy].Rev Med Inst Mex Seguro Soc. 2024 Jul 1;62(4):1-8. doi: 10.5281/zenodo.11397181. Rev Med Inst Mex Seguro Soc. 2024. PMID: 39542542 Free PMC article. Review. Spanish.
Cited by
-
Near-Infrared Fluorescent Endoscopic Image-Guided Photothermal Ablation Therapy of Colorectal Cancer Using Dual-Modal Gold Nanorods Targeting Tumor-Infiltrating Innate Immune Cells in a Transgenic TS4 CRE/APC loxΔ468 Mouse Model.ACS Appl Mater Interfaces. 2019 Jun 19;11(24):21353-21359. doi: 10.1021/acsami.9b04186. Epub 2019 Jun 4. ACS Appl Mater Interfaces. 2019. PMID: 31117445 Free PMC article.
-
Magnetic resonance imaging-guided and targeted theranostics of colorectal cancer.Cancer Biol Med. 2020 May 15;17(2):307-327. doi: 10.20892/j.issn.2095-3941.2020.0072. Cancer Biol Med. 2020. PMID: 32587771 Free PMC article. Review.
-
Image-Guided Cancer Nanomedicine.J Imaging. 2018;4(1):18. doi: 10.3390/jimaging4010018. Epub 2018 Jan 11. J Imaging. 2018. PMID: 30364459 Free PMC article.
-
Nanovectors Design for Theranostic Applications in Colorectal Cancer.J Oncol. 2019 Oct 1;2019:2740923. doi: 10.1155/2019/2740923. eCollection 2019. J Oncol. 2019. PMID: 31662751 Free PMC article. Review.
-
Multifunctional Nanocarriers-Mediated Synergistic Combination of Immune Checkpoint Inhibitor Cancer Immunotherapy and Interventional Oncology Therapy.Adv Nanobiomed Res. 2021 Oct;1(10):2100010. doi: 10.1002/anbr.202100010. Epub 2021 Aug 2. Adv Nanobiomed Res. 2021. PMID: 35663354 Free PMC article.
References
-
- American Cancer Society . Cancer facts & figures 2015. Atlanta, Ga: American Cancer Society, 2015.
-
- LeGolvan MP, Resnick M. Pathobiology of colorectal cancer hepatic metastases with an emphasis on prognostic factors. J Surg Oncol 2010;102(8):898–908. - PubMed
-
- Viganò L, Russolillo N, Ferrero A, Langella S, Sperti E, Capussotti L. Evolution of long-term outcome of liver resection for colorectal metastases: analysis of actual 5-year survival rates over two decades. Ann Surg Oncol 2012;19(6):2035–2044. - PubMed
-
- Brouquet A, Mortenson MM, Vauthey JN, et al. . Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg 2010;210(6):934–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

