Blocking endothelin-1-receptor/β-catenin circuit sensitizes to chemotherapy in colorectal cancer
- PMID: 28708138
- PMCID: PMC5596423
- DOI: 10.1038/cdd.2017.121
Blocking endothelin-1-receptor/β-catenin circuit sensitizes to chemotherapy in colorectal cancer
Abstract
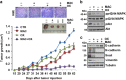
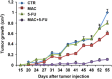
The limited clinical response to conventional chemotherapeutics observed in colorectal cancer (CRC) may be related to the connections between the hyperactivated β-catenin signaling and other pathways in CRC stem-like cells (CRC-SC). Here, we show the mechanistic link between the endothelin-1 (ET-1)/ET-1 receptor (ET-1R) signaling and β-catenin pathway through the specific interaction with the signal transducer β-arrestin1 (β-arr1), which initiates signaling cascades as part of the signaling complex. Using a panel of patient-derived CRC-SC, we show that these cells secrete ET-1 and express ETAR and β-arr1, and that the activation of ETAR/β-arr1 axis promotes the cross-talk with β-catenin signaling to sustain stemness, epithelial-to-mesenchymal transition (EMT) phenotype and response to chemotherapy. Upon ETAR activation, β-arr1 acts as a transcription co-activator that binds β-catenin, thereby promoting nuclear complex with β-catenin/TFC4 and p300 and histone acetylation, inducing chromatin reorganization on target genes, such as ET-1. The enhanced transcription of ET-1 increases the self-sustained ET-1/β-catenin network. All these findings provide a strong rationale for targeting ET-1R to hamper downstream β-catenin/ET-1 autocrine circuit. Interestingly, treatment with macitentan, a dual ETAR and ETBR antagonist, able to interfere with tumor and microenvironment, disrupts the ET-1R/β-arr1-β-catenin interaction impairing pathways involved in cell survival, EMT, invasion, and enhancing sensitivity to oxaliplatin (OX) and 5-fluorouracil (5-FU). In CRC-SC xenografts, the combination of macitentan and OX or 5-FU enhances the therapeutic effects of cytotoxic drugs. Together, these results provide mechanistic insight into how ET-1R coopts β-catenin signaling and offer a novel therapeutic strategy to manage CRC based on the combination of macitentan and chemotherapy that might benefit patients whose tumors show high ETAR and β-catenin expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006; 127: 469–480. - PubMed
-
- Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell 2014; 15: 692–705. - PubMed
-
- Colak S, Medema JP. Cancer stem cells-important players in tumor therapy resistance. FEBS J 2014; 281: 4779–4791. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

