Mifepristone Plasma Level and Glucocorticoid Receptor Antagonism Associated With Response in Patients With Psychotic Depression
- PMID: 28708736
- PMCID: PMC5596828
- DOI: 10.1097/JCP.0000000000000744
Mifepristone Plasma Level and Glucocorticoid Receptor Antagonism Associated With Response in Patients With Psychotic Depression
Abstract
Background: Psychotic depression has no Food and Drug Administration-approved treatment. Patients demonstrate significant dysregulation of the hypothalamic-pituitary-adrenal axis providing a biologically targeted treatment opportunity. The purpose of this study was to explore the clinical and biological effects of short-duration (7-day) glucocorticoid receptor antagonism with mifepristone and the role of mifepristone plasma levels in patients with psychotic depression.
Methods: This double-blind, randomized study took place at 34 US clinical research centers and included patients with a diagnosis of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, major depressive disorder, severe, with psychotic features. Patients underwent daily, observed, in-clinic administration of oral study drug (mifepristone 1200 mg or placebo) for days 1 to 7 of the 56-day trial, followed by treatment with a single Food and Drug Administration-approved antidepressant on days 8 to 56. The following scales were administered on days 0, 7, 14, 28, 42, and 56: Brief Psychiatric Rating Scale (BPRS), BPRS Positive Symptom Subscale, Hamilton Rating Scale for Depression, and Columbia-Suicide Severity Rating Scale. The primary end point was a categorical analysis evaluating the proportion of patients with 50% or greater reduction from baseline in BPRS Positive Symptom Subscale score on both days 7 and 56, demonstrating early and durable response. Cortisol and adrenocorticotropic hormone were measured on days 0, 7, 28, and 56. Mifepristone plasma levels were assessed on days 0 and 7.
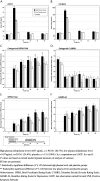
Results: An interim analysis indicated that the primary efficacy end point was unlikely to be met, and the study was stopped early with 292 of the planned 450 patients enrolled. Although the primary end point was not met, in a secondary prespecified analysis, patients who attained a mifepristone plasma level of 1637 ng/mL or greater (defined a priori and termed the high plasma level; 66.7% of patients) demonstrated statistically significant reductions in psychotic symptoms compared with patients who received placebo starting on day 28. This group also showed nonsignificant, numeric superiority on Hamilton Rating Scale for Depression improvement. No significant improvements were observed in the low-mifepristone group (<1637 ng/mL) versus the placebo group. There were no significant differences in Columbia-Suicide Severity Rating Scale suicidality ratings between groups.
Conclusions: Mifepristone 1200 mg daily for 7 days was safe and well tolerated, allowing most treated patients to achieve the a priori defined therapeutic plasma level of 1637 ng/mL, the mifepristone level associated with biological effect and clinical benefit.
Figures
References
-
- Ohayon MM, Schatzberg A. Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry. 2002;159:1855–1861. - PubMed
-
- Coryell W. Psychotic depression. J Clin Psychiatry. 1996;57(suppl 3):27–31. - PubMed
-
- Dubovsky SL, Thomas M. Psychotic depression: advances in conceptualization and treatment. Hosp Community Psychiatry. 1992;43:1189–1198. - PubMed
-
- Belanoff J, Kalehzan M, Sund B, et al. Cortisol activity and cognitive changes in psychotic major depression. Am J Psychiatry. 2001;158:1612–1616. - PubMed
-
- Johnson J, Horwath E, Weissman MM. The validity of major depression with psychotic features based on a community study. Arch Gen Psychiatry. 1991;48:1075–1081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous