Evolution of the Human Nervous System Function, Structure, and Development
- PMID: 28708995
- PMCID: PMC5647789
- DOI: 10.1016/j.cell.2017.06.036
Evolution of the Human Nervous System Function, Structure, and Development
Abstract
The nervous system-in particular, the brain and its cognitive abilities-is among humans' most distinctive and impressive attributes. How the nervous system has changed in the human lineage and how it differs from that of closely related primates is not well understood. Here, we consider recent comparative analyses of extant species that are uncovering new evidence for evolutionary changes in the size and the number of neurons in the human nervous system, as well as the cellular and molecular reorganization of its neural circuits. We also discuss the developmental mechanisms and underlying genetic and molecular changes that generate these structural and functional differences. As relevant new information and tools materialize at an unprecedented pace, the field is now ripe for systematic and functionally relevant studies of the development and evolution of human nervous system specializations.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
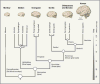
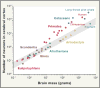
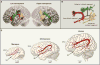
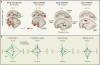
References
-
- Aiello LC, Wheeler P. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 1995;36:199–221.
-
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct. Funct. 2010;214:495–517. - PubMed
-
- Almécija S, Tallman M, Alba DM, Pina M, Moyá-Solá S, Jungers WL. The femur of Orrorin tugenensis exhibits morphometric affinities with both Miocene apes and later hominins. Nat. Commun. 2013;4:2888. - PubMed
-
- Alonso CR, Wilkins AS. The molecular elements that underlie developmental evolution. Nat. Rev. Genet. 2005;6:709–715. - PubMed
-
- Anderson JM, Gilmore R, Roper S, Crosson B, Bauer RM, Nadeau S, Beversdorf DQ, Cibula J, Rogish M, 3rd, Kortencamp S, et al. Conduction aphasia and the arcuate fasciculus: A reexamination of the Wernicke-Geschwind model. Brain Lang. 1999;70:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

