Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease
- PMID: 28708997
- PMCID: PMC5546158
- DOI: 10.1016/j.cell.2017.06.040
Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease
Abstract
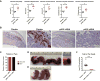
The emergence of Zika virus (ZIKV) and its association with congenital malformations has prompted the rapid development of vaccines. Although efficacy with multiple viral vaccine platforms has been established in animals, no study has addressed protection during pregnancy. We tested in mice two vaccine platforms, a lipid nanoparticle-encapsulated modified mRNA vaccine encoding ZIKV prM and E genes and a live-attenuated ZIKV strain encoding an NS1 protein without glycosylation, for their ability to protect against transmission to the fetus. Vaccinated dams challenged with a heterologous ZIKV strain at embryo day 6 (E6) and evaluated at E13 showed markedly diminished levels of viral RNA in maternal, placental, and fetal tissues, which resulted in protection against placental damage and fetal demise. As modified mRNA and live-attenuated vaccine platforms can restrict in utero transmission of ZIKV in mice, their further development in humans to prevent congenital ZIKV syndrome is warranted.
Keywords: Vaccine; antibody; fetus; flavivirus; immunity; microcephaly; pregnancy; transmission.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



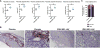
Similar articles
-
Zika Virus Attenuation by Codon Pair Deoptimization Induces Sterilizing Immunity in Mouse Models.J Virol. 2018 Aug 16;92(17):e00701-18. doi: 10.1128/JVI.00701-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925661 Free PMC article.
-
Vesicular Stomatitis Virus and DNA Vaccines Expressing Zika Virus Nonstructural Protein 1 Induce Substantial but Not Sterilizing Protection against Zika Virus Infection.J Virol. 2020 Aug 17;94(17):e00048-20. doi: 10.1128/JVI.00048-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32554698 Free PMC article.
-
Vaccine protection against Zika virus from Brazil.Nature. 2016 Aug 25;536(7617):474-8. doi: 10.1038/nature18952. Epub 2016 Jun 28. Nature. 2016. PMID: 27355570 Free PMC article.
-
[Advances in Zika vaccines].Wei Sheng Wu Xue Bao. 2017 Feb 4;57(2):188-96. Wei Sheng Wu Xue Bao. 2017. PMID: 29750481 Review. Chinese.
-
Potential targets for therapeutic intervention and structure based vaccine design against Zika virus.Eur J Med Chem. 2018 Aug 5;156:444-460. doi: 10.1016/j.ejmech.2018.07.014. Epub 2018 Jul 10. Eur J Med Chem. 2018. PMID: 30015077 Review.
Cited by
-
mRNA nanomedicine: Design and recent applications.Exploration (Beijing). 2022 Sep 19;2(6):20210217. doi: 10.1002/EXP.20210217. Online ahead of print. Exploration (Beijing). 2022. PMID: 36249890 Free PMC article. Review.
-
E90 subunit vaccine protects mice from Zika virus infection and microcephaly.Acta Neuropathol Commun. 2018 Aug 10;6(1):77. doi: 10.1186/s40478-018-0572-7. Acta Neuropathol Commun. 2018. PMID: 30097059 Free PMC article.
-
mRNA as a Therapeutics: Understanding mRNA Vaccines.Adv Pharm Bull. 2022 Mar;12(2):274-282. doi: 10.34172/apb.2022.028. Epub 2021 May 16. Adv Pharm Bull. 2022. PMID: 35620336 Free PMC article. Review.
-
mRNA Vaccine Protects against Zika Virus.Vaccines (Basel). 2021 Dec 10;9(12):1464. doi: 10.3390/vaccines9121464. Vaccines (Basel). 2021. PMID: 34960211 Free PMC article.
-
A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage.Nat Commun. 2017 Sep 22;8(1):676. doi: 10.1038/s41467-017-00737-8. Nat Commun. 2017. PMID: 28939807 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

