No added diagnostic value of non-phosphorylated tau fraction (p-taurel) in CSF as a biomarker for differential dementia diagnosis
- PMID: 28709448
- PMCID: PMC5513364
- DOI: 10.1186/s13195-017-0275-5
No added diagnostic value of non-phosphorylated tau fraction (p-taurel) in CSF as a biomarker for differential dementia diagnosis
Abstract
Background: The Alzheimer's disease (AD) cerebrospinal fluid (CSF) biomarkers Aβ1-42, t-tau, and p-tau181 overlap with other diseases. New tau modifications or epitopes, such as the non-phosphorylated tau fraction (p-taurel), may improve differential dementia diagnosis. The goal of this study is to investigate if p-taurel can improve the diagnostic performance of the AD CSF biomarker panel for differential dementia diagnosis.
Methods: The study population consisted of 45 AD, 45 frontotemporal lobar degeneration (FTLD), 45 dementia with Lewy bodies (DLB), and 21 Creutzfeldt-Jakob disease (CJD) patients, and 20 cognitively healthy controls. A substantial subset of the patients was pathology-confirmed. CSF levels of Aβ1-42, t-tau, p-tau181, and p-taurel were determined with commercially available single-analyte enzyme-linked immunosorbent assay (ELISA) kits. Diagnostic performance was evaluated by receiver operating characteristic (ROC) curve analyses, and area under the curve (AUC) values were compared using DeLong tests.
Results: The diagnostic performance of single markers as well as biomarker ratios was determined for each pairwise comparison of different dementia groups and controls. The addition of p-taurel to the AD biomarker panel decreased its diagnostic performance when discriminating non-AD, FTLD, and DLB from AD. As a single marker, p-taurel increased the diagnostic performance for CJD. No significant difference was found in AUC values with the addition of p-taurel when differentiating between AD or non-AD dementias and controls.
Conclusions: The addition of p-taurel to the AD CSF biomarker panel failed to improve differentiation between AD and non-AD dementias.
Keywords: Alzheimer’s disease; Biomarkers; Cerebrospinal fluid; Creutzfeldt-Jakob disease; Dementia with Lewy bodies; Differential diagnosis; Frontotemporal lobar degeneration; Tau.
Figures
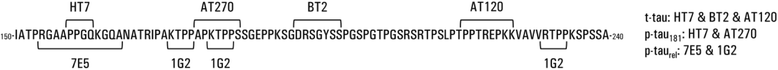

Similar articles
-
The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias.Alzheimers Res Ther. 2018 Jan 11;10(1):3. doi: 10.1186/s13195-017-0331-1. Alzheimers Res Ther. 2018. PMID: 29368621 Free PMC article.
-
Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort.Neurology. 2012 Jan 3;78(1):47-54. doi: 10.1212/WNL.0b013e31823ed0f0. Epub 2011 Dec 14. Neurology. 2012. PMID: 22170879
-
Cerebrospinal fluid in the differential diagnosis of Alzheimer's disease: clinical utility of an extended panel of biomarkers in a specialist cognitive clinic.Alzheimers Res Ther. 2018 Mar 20;10(1):32. doi: 10.1186/s13195-018-0361-3. Alzheimers Res Ther. 2018. PMID: 29558979 Free PMC article.
-
Tau and p-tau as CSF biomarkers in dementia: a meta-analysis.Clin Chem Lab Med. 2011 Mar;49(3):353-66. doi: 10.1515/CCLM.2011.086. Epub 2011 Feb 23. Clin Chem Lab Med. 2011. PMID: 21342021 Review.
-
Cerebrospinal fluid tau and beta-amyloid(1-42) in dementia disorders.Mech Ageing Dev. 2001 Nov;122(16):2005-11. doi: 10.1016/s0047-6374(01)00304-9. Mech Ageing Dev. 2001. PMID: 11589918 Review.
Cited by
-
Cognitive profiles in persons with depressive disorder and Alzheimer's disease.Brain Commun. 2020 Nov 27;2(2):fcaa206. doi: 10.1093/braincomms/fcaa206. eCollection 2020. Brain Commun. 2020. PMID: 33409492 Free PMC article.
-
Relationship Between Amyloid-β Deposition and Blood-Brain Barrier Dysfunction in Alzheimer's Disease.Front Cell Neurosci. 2021 Jul 19;15:695479. doi: 10.3389/fncel.2021.695479. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34349624 Free PMC article. Review.
-
Longitudinal chemokine profile expression in a blood-brain barrier model from Alzheimer transgenic versus wild-type mice.J Neuroinflammation. 2018 Jun 13;15(1):182. doi: 10.1186/s12974-018-1220-7. J Neuroinflammation. 2018. PMID: 29898739 Free PMC article.
-
On the conundrum of cognitive impairment due to depressive disorder in older patients.PLoS One. 2020 Apr 2;15(4):e0231111. doi: 10.1371/journal.pone.0231111. eCollection 2020. PLoS One. 2020. PMID: 32240257 Free PMC article.
-
Cerebrospinal fluid non-phosphorylated tau in the differential diagnosis of Creutzfeldt-Jakob disease: a comparative prospective study with 14-3-3.J Neurol. 2020 Feb;267(2):543-550. doi: 10.1007/s00415-019-09610-8. Epub 2019 Nov 7. J Neurol. 2020. PMID: 31701333
References
-
- Somers C, Struyfs H, Goossens J, Niemantsverdriet E, Luyckx J, De Roeck N, De Roeck E, De Vil B, Cras P, Martin J-J, De Deyn P-P, Bjerke M, Engelborghs S. A decade of cerebrospinal fluid biomarkers for Alzheimer’s disease in Belgium. J Alzheimers Dis. 2016;54:383–95. doi: 10.3233/JAD-151097. - DOI - PubMed
-
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert M-O, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29. doi: 10.1016/S1474-4422(14)70090-0. - DOI - PubMed
-
- Blennow K, Hampel H: CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

