Influence of the patient-practitioner interaction context on acupuncture outcomes in functional dyspepsia: study protocol for a multicenter randomized controlled trial
- PMID: 28709452
- PMCID: PMC5513038
- DOI: 10.1186/s12906-017-1869-y
Influence of the patient-practitioner interaction context on acupuncture outcomes in functional dyspepsia: study protocol for a multicenter randomized controlled trial
Abstract
Background: In the treatment of functional dyspepsia, the placebo effect has been reported to be high, and the influence of the patient-practitioner relationship may be a major component of this effect. The specific and non-specific effects of acupuncture cannot be easily distinguished, and the patient-practitioner relationship may influence the total therapeutic effect in clinical practice. There have been no studies that investigate the influence of patient-practitioner relationship on acupuncture treatment for patients with functional dyspepsia.
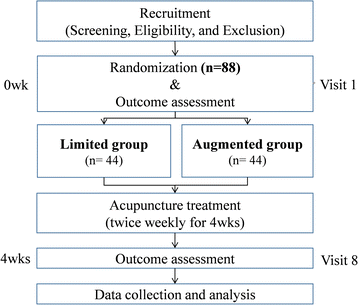
Methods: Patients with postprandial distress syndrome, a functional dyspepsia subtype, will be recruited at three hospitals (two in Korea and one in USA) for an international, multi-center, randomized, patient/assessor-blinded, clinical trial. The total anticipated sample size is 88. The participants will be randomly allocated into two groups: an augmented interaction group and a limited interaction group. Acupuncture, with total 12 acupoints, will be performed twice weekly for 4 weeks in both groups. Trained practitioners will provide an "augmented" or "limited" interaction context, as determined by random allocation. The primary outcome measure is the proportion of responders, the proportion of participants who answer "yes" to more than half of the adequate relief questions during the study. Secondary outcome measures include questionnaires for quality of life and symptoms of dyspepsia, and maximum tolerable volume of nutrient drink test. Data will be collected at baseline and following 4 weeks of acupuncture.
Discussion: This study will evaluate the influence of the patient-practitioner interaction on clinical effects of acupuncture in patients with functional dyspepsia.
Trial registration: CRIS Identifier: ( KCT0002229 ).
Keywords: Acupuncture; Augmented interaction; Functional dyspepsia; Limited interaction; Randomized controlled trial.
Conflict of interest statement
Ethics approval and consent to participate
This trial will be carried out according to the standards of the International Committee on Harmonization on Good Clinical Practice and the Declaration of Helsinki. Medical Ethics Committee at KyungHee University Hospital at Gangdong (approval number is KHNMCOH 2016-04-007-001), Kyung Hee University Medical Center (No. KOMCIRB – 150914 – HR - 040), and Massachusetts General Hospital (No. 2016P001981/PHS) have approved this protocol. Informed consent will be gained from all participants before commencing the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Similar articles
-
Effect of acupuncture and its influence on cerebral activity in functional dyspepsia patients: study protocol for a randomized controlled trial.Trials. 2016 Apr 2;17:183. doi: 10.1186/s13063-016-1296-2. Trials. 2016. PMID: 27039086 Free PMC article. Clinical Trial.
-
Acupuncture for functional dyspepsia: study protocol for a two-center, randomized controlled trial.Trials. 2014 Mar 22;15:89. doi: 10.1186/1745-6215-15-89. Trials. 2014. PMID: 24655542 Free PMC article. Clinical Trial.
-
Acupuncture for postprandial distress syndrome (APDS): study protocol for a randomized controlled trial.Trials. 2017 Nov 13;18(1):537. doi: 10.1186/s13063-017-2285-9. Trials. 2017. PMID: 29132415 Free PMC article. Clinical Trial.
-
Which subtype of functional dyspepsia patients responses better to acupuncture? A retrospective analysis of a randomized controlled trial.Forsch Komplementmed. 2015;22(2):94-100. doi: 10.1159/000380983. Epub 2015 Mar 11. Forsch Komplementmed. 2015. PMID: 26021959 Review.
-
[Guideline Recommendation for Endpoints Used in Clinical Trials for Functional Dyspepsia].Korean J Gastroenterol. 2018 Oct 25;72(4):170-178. doi: 10.4166/kjg.2018.72.4.170. Korean J Gastroenterol. 2018. PMID: 30419642 Review. Korean.
Cited by
-
Individual Differences in Responsiveness to Acupuncture: An Exploratory Survey of Practitioner Opinion.Medicines (Basel). 2018 Aug 6;5(3):85. doi: 10.3390/medicines5030085. Medicines (Basel). 2018. PMID: 30082630 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical