Effects of inhaled hypertonic (7%) saline on lung function test in preschool children with cystic fibrosis: results of a crossover, randomized clinical trial
- PMID: 28709466
- PMCID: PMC5512793
- DOI: 10.1186/s13052-017-0376-6
Effects of inhaled hypertonic (7%) saline on lung function test in preschool children with cystic fibrosis: results of a crossover, randomized clinical trial
Abstract
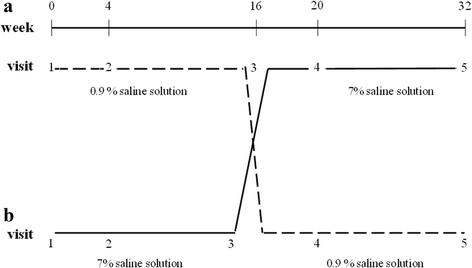
Background: This crossover, randomized, double-blind study (conducted over a 32-week period) was performed to determine, in clinically stable Cystic fibrosis (CF) preschool children: the effects of 7% inhaled hypertonic saline on spirometry and interrupter resistance technique (Rint), and the possible side effects.
Methods: Twelve CF children (6M, mean age ± SD: 5.7 ± 0.8 yrs) were enrolled and randomly assigned to receive hypertonic saline (HS-4 ml 7% sodium chloride), or normal saline (NS-0.9% sodium chloride) twice a day. After a 16 weeks period, therapy was exchanged to allow all the patients enrolled in the study to carry out both treatments. Monitoring visits, spirometry (COSMED Quark PFT4 ergo) and Rint were scheduled at 0,4,16,20,32 weeks. At T0, spirometric measurements and Rint were performed immediately before and 30 min after the inhalation therapy. Salbutamol (400 mcg) was administered before the drug at each visit.
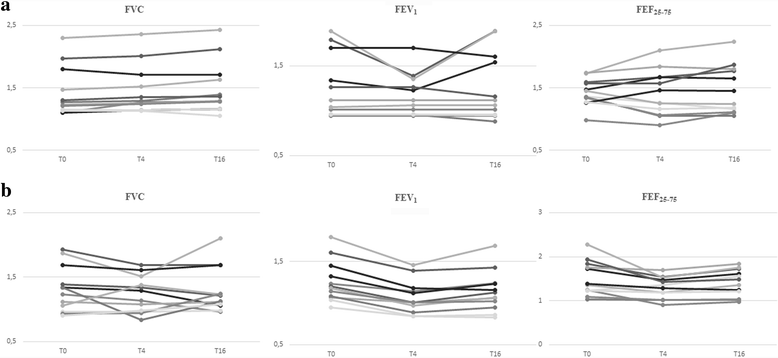
Results: After a 16-weeks treatment with HS an improvement of FVC (p = 0.02) and a favorable trend of FEV1 were registered. A worsening of FEV1 (p < 0.0001) and of FEF25-75 (p = 0.019) were found in NS group. No differences were found in expiratory and inspiratory Rint in both groups. No serious adverse events occurred.
Conclusions: Seven percent hypertonic saline therapy proved to be a useful and safe treatment in young CF children with clinically stable conditions.
Trial registration: ISRCTN12345678 .
Keywords: Children; Cystic fibrosis; Hypertonic saline; Inhalation; Therapy.
Conflict of interest statement
Ethics approval and consent to participate
All parents of children gave written informed consent and the protocol (prot. HS-2009) was approved by the Scientific Ethics Committee, Policlinico Umberto I.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Wark P, Mc Donald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Sist Rev. 2009;15:CD001506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous