Interaction of the innate immune system with positive-strand RNA virus replication organelles
- PMID: 28709747
- PMCID: PMC7108334
- DOI: 10.1016/j.cytogfr.2017.05.007
Interaction of the innate immune system with positive-strand RNA virus replication organelles
Abstract
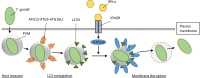
The potential health risks associated with (re-)emerging positive-strand RNA (+RNA) viruses emphasizes the need for understanding host-pathogen interactions for these viruses. The innate immune system forms the first line of defense against pathogenic organisms like these and is responsible for detecting pathogen-associated molecular patterns (PAMPs). Viral RNA is a potent inducer of antiviral innate immune signaling, provoking an antiviral state by directing expression of interferons (IFNs) and pro-inflammatory cytokines. However, +RNA viruses developed various methods to avoid detection and downstream signaling, including isolation of viral RNA replication in membranous viral replication organelles (ROs). These structures therefore play a central role in infection, and consequently, loss of RO integrity might simultaneously result in impaired viral replication and enhanced antiviral signaling. This review summarizes the first indications that the innate immune system indeed has tools to disrupt viral ROs and other non- or aberrant-self membrane structures, and may do this by marking these membranes with proteins such as microtubule-associated protein 1A/1B-light chain 3 (LC3) and ubiquitin, resulting in the recruitment of IFN-inducible GTPases. Further studies should evaluate whether this process forms a general effector mechanism in +RNA virus infection, thereby creating the opportunity for development of novel antiviral therapies.
Keywords: +RNA virus; GTPases; Interferon; Replication organelles.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

