An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal γδ T Cells
- PMID: 28709803
- PMCID: PMC5553552
- DOI: 10.1016/j.immuni.2017.06.014
An Ocular Commensal Protects against Corneal Infection by Driving an Interleukin-17 Response from Mucosal γδ T Cells
Abstract
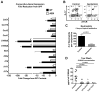
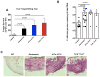
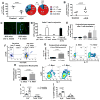
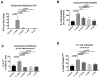
Mucosal sites such as the intestine, oral cavity, nasopharynx, and vagina all have associated commensal flora. The surface of the eye is also a mucosal site, but proof of a living, resident ocular microbiome remains elusive. Here, we used a mouse model of ocular surface disease to reveal that commensals were present in the ocular mucosa and had functional immunological consequences. We isolated one such candidate commensal, Corynebacterium mastitidis, and showed that this organism elicited a commensal-specific interleukin-17 response from γδ T cells in the ocular mucosa that was central to local immunity. The commensal-specific response drove neutrophil recruitment and the release of antimicrobials into the tears and protected the eye from pathogenic Candida albicans or Pseudomonas aeruginosa infection. Our findings provide direct evidence that a resident commensal microbiome exists on the ocular surface and identify the cellular mechanisms underlying its effects on ocular immune homeostasis and host defense.
Keywords: IL-17; host defense; microbiome; mucosal immunity; ocular commensal bacteria; ocular surface disease; γδ T cells.
Published by Elsevier Inc.
Figures


Comment in
-
A Commencement for Eye Commensals.Immunity. 2017 Jul 18;47(1):6-8. doi: 10.1016/j.immuni.2017.07.006. Immunity. 2017. PMID: 28723553 Free PMC article.
-
Mucosal immunology: Eye spy a protective commensal.Nat Rev Immunol. 2017 Sep;17(9):531. doi: 10.1038/nri.2017.92. Epub 2017 Aug 7. Nat Rev Immunol. 2017. PMID: 28787400 No abstract available.
Similar articles
-
The microbiota protects against Pseudomonas aeruginosa pneumonia via γδ T cell-neutrophil axis in mice.Microbes Infect. 2020 Sep;22(8):294-302. doi: 10.1016/j.micinf.2020.04.003. Epub 2020 Apr 17. Microbes Infect. 2020. PMID: 32305502
-
IL-17 plays a central role in initiating experimental Candida albicans infection in mouse corneas.Eur J Immunol. 2013 Oct;43(10):2671-82. doi: 10.1002/eji.201242891. Epub 2013 Aug 12. Eur J Immunol. 2013. PMID: 23843112
-
Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis.J Immunol. 2014 Feb 15;192(4):1745-52. doi: 10.4049/jimmunol.1302265. Epub 2014 Jan 17. J Immunol. 2014. PMID: 24442441 Free PMC article.
-
IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans.J Immunol. 2015 Aug 1;195(3):780-8. doi: 10.4049/jimmunol.1500909. J Immunol. 2015. PMID: 26188072 Free PMC article. Review.
-
IL-17 signaling in host defense against Candida albicans.Immunol Res. 2011 Aug;50(2-3):181-7. doi: 10.1007/s12026-011-8226-x. Immunol Res. 2011. PMID: 21717069 Free PMC article. Review.
Cited by
-
Alterations in the Ocular Surface Microbiome in Traumatic Corneal Ulcer Patients.Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):35. doi: 10.1167/iovs.61.6.35. Invest Ophthalmol Vis Sci. 2020. PMID: 32543662 Free PMC article.
-
Association of Single-Nucleotide Polymorphisms in Interleukin Genes with Microbial Keratitis in a South Indian Population.Pathogens. 2022 Nov 20;11(11):1387. doi: 10.3390/pathogens11111387. Pathogens. 2022. PMID: 36422638 Free PMC article.
-
Neutrophils cause obstruction of eyelid sebaceous glands in inflammatory eye disease in mice.Sci Transl Med. 2018 Jul 25;10(451):eaas9164. doi: 10.1126/scitranslmed.aas9164. Sci Transl Med. 2018. PMID: 30045980 Free PMC article.
-
Impact of Exposomes on Ocular Surface Diseases.Int J Mol Sci. 2023 Jul 10;24(14):11273. doi: 10.3390/ijms241411273. Int J Mol Sci. 2023. PMID: 37511032 Free PMC article. Review.
-
Bacteria and Dry Eye: A Narrative Review.J Clin Med. 2022 Jul 12;11(14):4019. doi: 10.3390/jcm11144019. J Clin Med. 2022. PMID: 35887783 Free PMC article. Review.
References
-
- Agnifili L, Mastropasqua R, Fasanella V, Di Staso S, Mastropasqua A, Brescia L, Mastropasqua L. In vivo confocal microscopy of conjunctiva-associated lymphoid tissue in healthy humans. Invest Ophthalmol Vis Sci. 2014;55:5254–5262. - PubMed
-
- Bernard KA, Pacheco AL, Loomer C, Burdz T, Wiebe D, Huyhn C, Kaplen B, Olson AB, Cnockaert M, Eguchi H, et al. Corynebacterium lowii sp. nov. and Corynebacterium oculi sp. nov., derived from Human Clinical Disease and an Emended Description of Corynebacterium mastitidis. Int J Syst Evol Microbiol 2016 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

