The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus
- PMID: 28709914
- PMCID: PMC5673570
- DOI: 10.1016/j.clim.2017.07.004
The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus
Abstract
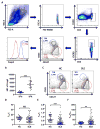
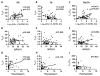
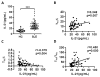
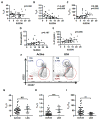
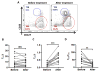
Follicular T regulatory (Tfr) cells inhibit follicular T helper (Tfh) cells mediated B cell responses. Tfh cells are involved in the pathogenesis of systemic lupus erythematosus (SLE). However, the role of Tfr cells in SLE remains unclear. The frequency of circulating Tfr and Tfh cells were examined in SLE patients and healthy controls. The frequency of circulating Tfr cell decreased and Tfh/Tfr ratio increased in SLE patients. Serum anti-dsDNA antibody level positively correlated with frequency of Tfh cells and Tfh/Tfr ratios but negatively correlated with the frequency of Tfr cells. Moreover, the frequency of Tfr and Tfh/Tfr ratio but not that of Tfh was correlated with diseases activity. In addition, increase in Tfr cell numbers and decrease in the Tfh/Tfr ratios were observed with successful treatments. Thus, Tfr cells should be considered as a biomarker for SLE and their role in the pathogenesis of SLE warrants further investigation.
Keywords: Biomarker; SLE; Tfh; Tfr.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
All the authors have no interests to declare.
Figures

References
-
- Blanco P, Ueno H, Schmitt N. T follicular helper (Tfh) cells in lupus: activation and involvement in SLE pathogenesis. Eur J Immunol. 2016;46(2):281–290. - PubMed
-
- D’Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369(9561):587–596. - PubMed
-
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–2121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

