Gq-activated fibroblasts induce cardiomyocyte action potential prolongation and automaticity in a three-dimensional microtissue environment
- PMID: 28710068
- PMCID: PMC5668610
- DOI: 10.1152/ajpheart.00181.2017
Gq-activated fibroblasts induce cardiomyocyte action potential prolongation and automaticity in a three-dimensional microtissue environment
Abstract
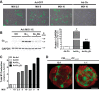
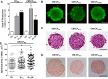
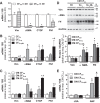
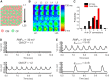
Cardiac fibroblasts (CFs) are known to regulate cardiomyocyte (CM) function in vivo and in two-dimensional in vitro cultures. This study examined the effect of CF activation on the regulation of CM electrical activity in a three-dimensional (3-D) microtissue environment. Using a scaffold-free 3-D platform with interspersed neonatal rat ventricular CMs and CFs, Gq-mediated signaling was selectively enhanced in CFs by Gαq adenoviral infection before coseeding with CMs in nonadhesive hydrogels. After 3 days, the microtissues were analyzed by signaling assay, histological staining, quantitative PCR, Western blots, optical mapping with voltage- or Ca2+-sensitive dyes, and microelectrode recordings of CF resting membrane potential (RMPCF). Enhanced Gq signaling in CFs increased microtissue size and profibrotic and prohypertrophic markers. Expression of constitutively active Gαq in CFs prolonged CM action potential duration (by 33%) and rise time (by 31%), prolonged Ca2+ transient duration (by 98%) and rise time (by 65%), and caused abnormal electrical activity based on depolarization-induced automaticity. Constitutive Gq activation in CFs also depolarized RMPCF from -33 to -20 mV and increased connexin 43 and connexin 45 expression. Computational modeling confers that elevated RMPCF and increased cell-cell coupling between CMs and CFs in a 3-D environment could lead to automaticity. In conclusion, our data demonstrate that CF activation alone is capable of altering action potential and Ca2+ transient characteristics of CMs, leading to proarrhythmic electrical activity. Our results also emphasize the importance of a 3-D environment where cell-cell interactions are prevalent, underscoring that CF activation in 3-D tissue plays a significant role in modulating CM electrophysiology and arrhythmias.NEW & NOTEWORTHY In a three-dimensional microtissue model, which lowers baseline activation of cardiac fibroblasts but enables cell-cell, paracrine, and cell-extracellular matrix interactions, we demonstrate that selective cardiac fibroblast activation by enhanced Gq signaling, a pathophysiological trigger in the diseased heart, modulates cardiomyocyte electrical activity, leading to proarrhythmogenic automaticity.
Keywords: activated cardiac fibroblasts; arrhythmias; automaticity; cardiac myocytes; three-dimensional models.
Copyright © 2017 the American Physiological Society.
Figures





Similar articles
-
Directed fusion of cardiac spheroids into larger heterocellular microtissues enables investigation of cardiac action potential propagation via cardiac fibroblasts.PLoS One. 2018 May 1;13(5):e0196714. doi: 10.1371/journal.pone.0196714. eCollection 2018. PLoS One. 2018. PMID: 29715271 Free PMC article.
-
Functional scaffold-free 3-D cardiac microtissues: a novel model for the investigation of heart cells.Am J Physiol Heart Circ Physiol. 2012 May 15;302(10):H2031-42. doi: 10.1152/ajpheart.00743.2011. Epub 2012 Mar 16. Am J Physiol Heart Circ Physiol. 2012. PMID: 22427522 Free PMC article.
-
Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes.Cardiovasc Res. 2009 Sep 1;83(4):688-97. doi: 10.1093/cvr/cvp164. Epub 2009 May 28. Cardiovasc Res. 2009. PMID: 19477968 Free PMC article.
-
Electrical consequences of cardiac myocyte: fibroblast coupling.Biochem Soc Trans. 2015 Jun;43(3):513-8. doi: 10.1042/BST20150035. Biochem Soc Trans. 2015. PMID: 26009200 Review.
-
Actions of emigrated neutrophils on Na(+) and K(+) currents in rat ventricular myocytes.Prog Biophys Mol Biol. 2006 Jan-Apr;90(1-3):249-69. doi: 10.1016/j.pbiomolbio.2005.07.003. Epub 2005 Aug 9. Prog Biophys Mol Biol. 2006. PMID: 16165196 Review.
Cited by
-
Phenotypic Variation Between Stromal Cells Differentially Impacts Engineered Cardiac Tissue Function.Tissue Eng Part A. 2019 May;25(9-10):773-785. doi: 10.1089/ten.TEA.2018.0362. Tissue Eng Part A. 2019. PMID: 30968748 Free PMC article.
-
Human myofibroblasts increase the arrhythmogenic potential of human induced pluripotent stem cell-derived cardiomyocytes.Cell Mol Life Sci. 2023 Sep 5;80(9):276. doi: 10.1007/s00018-023-04924-3. Cell Mol Life Sci. 2023. PMID: 37668685 Free PMC article.
-
Directed fusion of cardiac spheroids into larger heterocellular microtissues enables investigation of cardiac action potential propagation via cardiac fibroblasts.PLoS One. 2018 May 1;13(5):e0196714. doi: 10.1371/journal.pone.0196714. eCollection 2018. PLoS One. 2018. PMID: 29715271 Free PMC article.
-
Engineering and Assessing Cardiac Tissue Complexity.Int J Mol Sci. 2021 Feb 2;22(3):1479. doi: 10.3390/ijms22031479. Int J Mol Sci. 2021. PMID: 33540699 Free PMC article. Review.
-
Modeling cardiac complexity: Advancements in myocardial models and analytical techniques for physiological investigation and therapeutic development in vitro.APL Bioeng. 2019 Feb 5;3(1):011501. doi: 10.1063/1.5055873. eCollection 2019 Mar. APL Bioeng. 2019. PMID: 31069331 Free PMC article. Review.
References
-
- Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Müller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 115: 625–635, 2014. doi:10.1161/CIRCRESAHA.115.303794. - DOI - PubMed
-
- Askar SF, Bingen BO, Swildens J, Ypey DL, van der Laarse A, Atsma DE, Zeppenfeld K, Schalij MJ, de Vries AA, Pijnappels DA. Connexin43 silencing in myofibroblasts prevents arrhythmias in myocardial cultures: role of maximal diastolic potential. Cardiovasc Res 93: 434–444, 2012. doi:10.1093/cvr/cvr351. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

