Manipulation of a Senescence-Associated Gene Improves Fleshy Fruit Yield
- PMID: 28710129
- PMCID: PMC5580748
- DOI: 10.1104/pp.17.00452
Manipulation of a Senescence-Associated Gene Improves Fleshy Fruit Yield
Abstract
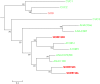
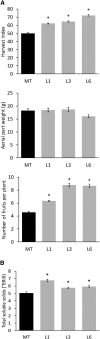
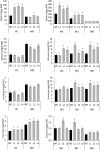
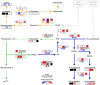
Senescence is the process that marks the end of a leaf's lifespan. As it progresses, the massive macromolecular catabolism dismantles the chloroplasts and, consequently, decreases the photosynthetic capacity of these organs. Thus, senescence manipulation is a strategy to improve plant yield by extending the leaf's photosynthetically active window of time. However, it remains to be addressed if this approach can improve fleshy fruit production and nutritional quality. One way to delay senescence initiation is by regulating key transcription factors (TFs) involved in triggering this process, such as the NAC TF ORESARA1 (ORE1). Here, three senescence-related NAC TFs from tomato (Solanum lycopersicum) were identified, namely SlORE1S02, SlORE1S03, and SlORE1S06. All three genes were shown to be responsive to senescence-inducing stimuli and posttranscriptionally regulated by the microRNA miR164 Moreover, the encoded proteins interacted physically with the chloroplast maintenance-related TF SlGLKs. This characterization led to the selection of a putative tomato ORE1 as target gene for RNA interference knockdown. Transgenic lines showed delayed senescence and enhanced carbon assimilation that, ultimately, increased the number of fruits and their total soluble solid content. Additionally, the fruit nutraceutical composition was enhanced. In conclusion, these data provide robust evidence that the manipulation of leaf senescence is an effective strategy for yield improvement in fleshy fruit-bearing species.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures






References
-
- Almeida J, Asís R, Molineri VN, Sestari I, Lira BS, Carrari F, Peres LEP, Rossi M (2015) Fruits from ripening impaired, chlorophyll degraded and jasmonate insensitive tomato mutants have altered tocopherol content and composition. Phytochemistry 111: 72–83 - PubMed
-
- Almeida J, Azevedo MdaS, Spicher L, Glauser G, vom Dorp K, Guyer L, del Valle Carranza A, Asis R, de Souza AP, Buckeridge M, et al. (2016) Down-regulation of tomato PHYTOL KINASE strongly impairs tocopherol biosynthesis and affects prenyllipid metabolism in an organ-specific manner. J Exp Bot 67: 919–934 - PMC - PubMed
-
- Balazadeh S, Riaño-Pachón DM, Mueller-Roeber B (2008) Transcription factors regulating leaf senescence in Arabidopsis thaliana. Plant Biol (Stuttg) (Suppl 1) 10: 63–75 - PubMed
-
- Balazadeh S, Siddiqui H, Allu AD, Matallana-Ramirez LP, Caldana C, Mehrnia M, Zanor MI, Köhler B, Mueller-Roeber B (2010) A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J 62: 250–264 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

