Perspective: Neuroregenerative Nutrition
- PMID: 28710142
- PMCID: PMC5502873
- DOI: 10.3945/an.117.015388
Perspective: Neuroregenerative Nutrition
Abstract
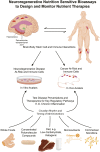
Good health while aging depends upon optimal cellular and organ functioning that contribute to the regenerative ability of the body during the lifespan, especially when injuries and diseases occur. Although diet may help in the maintenance of cellular fitness during periods of stability or modest decline in the regenerative function of an organ, this approach is inadequate in an aged system, in which the ability to maintain homeostasis is further challenged by aging and the ensuing suboptimal functioning of the regenerative unit, tissue-specific stem cells. Focused nutritional approaches can be used as an intervention to reduce decline in the body's regenerative capacity. This article brings together nutrition-associated therapeutic approaches with the fields of aging, immunology, neurodegenerative disease, and cancer to propose ways in which diet and nutrition can work with standard-of-care and integrated medicine to help improve the brain's function as it ages. The field of regenerative medicine has exploded during the past 2 decades as a result of the discovery of stem cells in nearly every organ system of the body, including the brain, where neural stem cells persist in discrete areas throughout life. This fact, and the uncovering of the genetic basis of plasticity in somatic cells and cancer stem cells, open a door to a world where maintenance and regeneration of organ systems maintain health and extend life expectancy beyond its present limits. An area that has received little attention in regenerative medicine is the influence on regulatory mechanisms and therapeutic potential of nutrition. We propose that a strong relation exists between brain regenerative medicine and nutrition and that nutritional intervention at key times of life could be used to not only maintain optimal functioning of regenerative units as humans age but also play a primary role in therapeutic treatments to combat injury and diseases (in particular, those that occur in the latter one-third of the lifespan).
Keywords: age- and disease-related inflammation; avatar models; combination nutrient therapies; nutrition; regenerative medicine; stem cells.
© 2017 American Society for Nutrition.
Conflict of interest statement
Author disclosures: The authors are affiliated with a new start-up company, Prana Therapeutics, Inc. They have received no financial compensation in relation to this article.
Figures
References
-
- Coras R, Siebzehnrubl FA, Pauli E, Huttner HB, Njunting M, Kobow K, Villmann C, Hahnen E, Neuhuber W, Weigel D, et al. . Low proliferation and differentiation capacities of adult hippocampal stem cells correlate with memory dysfunction in humans. Brain 2010;133:3359–72. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

