Regulation of Bone Metabolism by Sex Steroids
- PMID: 28710257
- PMCID: PMC5749141
- DOI: 10.1101/cshperspect.a031211
Regulation of Bone Metabolism by Sex Steroids
Abstract
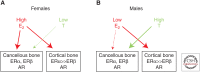
Osteoporosis is a significant public health problem, and a major cause of the disease is estrogen deficiency following menopause in women. In addition, considerable evidence now shows that estrogen is also a major regulator of bone metabolism in men. Since the original description of the effects of estrogen deficiency on bone by Fuller Albright more than 70 years ago, there has been enormous progress in understanding the mechanisms of estrogen and testosterone action on bone using human and mouse models. Although we understand more about the effects of estrogen on bone as compared with testosterone, both sex steroids do play important roles, perhaps in a somewhat compartment-specific (i.e., cancellous vs. cortical bone) manner. This review summarizes our current knowledge of sex steroid action on bone based on human and mouse studies, identifies both agreements and potential discrepancies between these studies, and suggests directions for future research in this important area.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Albright F. 1940. Post-menopausal osteoporosis. Trans Assoc Am Physicians 55: 298–305.
-
- Bilezikian JP, Morishima A, Bell J, Grumbach MM. 1998. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med 339: 599–603. - PubMed
-
- Bonnick SL. 1998. Skeletal anatomy in densitometry. In Bone densitometry in clinical practice (ed. Bonnick SL), pp. 35–78. Humana, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
