Open-label trial of ranolazine for the treatment of myotonia congenita
- PMID: 28710329
- PMCID: PMC5562961
- DOI: 10.1212/WNL.0000000000004229
Open-label trial of ranolazine for the treatment of myotonia congenita
Abstract
Objective: To determine open-label, pilot study whether ranolazine could improve signs and symptoms of myotonia and muscle stiffness in patients with myotonia congenita (MC).
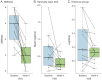
Methods: Thirteen participants were assessed at baseline and 2, 4, and 5 weeks. Ranolazine was started after baseline assessment (500 mg twice daily), increased as tolerated after week 2 (1,000 mg twice daily), and maintained until week 4. Outcomes included change from baseline to week 4 in self-reported severity of symptoms (stiffness, weakness, and pain), Timed Up and Go (TUG), hand grip and eyelid myotonia, and myotonia on EMG.
Results: Self-reported severity of stiffness (p < 0.0001) and weakness (p < 0.01) was significantly improved compared with baseline. TUG and grip myotonia times were reduced (p = 0.03, p = 0.01). EMG of the abductor digiti minimi and tibialis anterior showed significantly reduced myotonia duration (p < 0.001, p < 0.01) at week 4. No participant discontinued ranolazine because of side effects.
Conclusions: Ranolazine appeared to be well tolerated over a period of 4 weeks in individuals with MC, and ranolazine resulted in improvement of signs and symptoms of muscle stiffness. The findings of this study suggest that ranolazine should be investigated in a larger controlled study.
Classification of evidence: This study provides Class IV evidence that ranolazine improves myotonia in myotonia congenita.
© 2017 American Academy of Neurology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials