Vincristine, Ifosfamide, and Doxorubicin for Initial Treatment of Ewing Sarcoma in Adults
- PMID: 28710342
- PMCID: PMC5634776
- DOI: 10.1634/theoncologist.2016-0464
Vincristine, Ifosfamide, and Doxorubicin for Initial Treatment of Ewing Sarcoma in Adults
Abstract
Background: There are no clinical trials specifically addressing chemotherapy for adults with Ewing sarcoma (ES). Five-year event-free survival (EFS) of adults on pediatric studies of ES (44%-47%) is worse than that of children treated with the same therapy (69%). The object of this study was to review the results of therapy with vincristine, ifosfamide, and doxorubicin (VID) in the multidisciplinary treatment of adults with ES at our institution.
Materials and methods: Charts for adults treated for ES from 1995 to 2011 were retrospectively reviewed. Clinician-reported radiographic tumor response, type of local therapy, pathologic response, and survival data were collected.
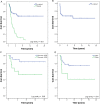
Results: Seventy-one patients were identified who received VID as initial therapy. The median age was 25 (range: 16-64). Forty-two patients (59%) presented with a localized disease and 29 patients (41%) presented with a distant metastasis. Of all patients treated with VID, 83.6% showed a radiological response. Patients who presented with a localized disease had a 5-year overall survival (OS) of 68% (median not reached), compared with 10.3% (median: 1.9 years) in those who presented with distant metastases. Five-year EFS was 67%. The nine patients with a pelvic primary tumor had inferior 5-year OS (42%) to the 33 with primary tumors at other sites (75%). The 5-year OS of those who had greater than or equal to 95% necrosis after neoadjuvant VID (n = 20; 5-year OS: 84%) was superior to those who had less than 95% necrosis (n = 13; 5-year OS: 53%).
Conclusion: In adults with primary ES, VID combined with an adjuvant strategy based on post-treatment percent necrosis has favorable outcomes compared with historical adult controls.
Implications for practice: Ewing sarcoma (ES) is a rare tumor in adults, and there are no dedicated clinical trials in the adult population. Most therapy is modeled after the published pediatric studies, although the small numbers of adult patients included on those studies did significantly worse than the children. We modeled our treatment on other adult sarcomas and reviewed the charts of 71 adult patients with ES treated with vincristine, ifosfamide, and doxorubicin (VID). In adults with primary ES, VID combined with an adjuvant strategy based on post-treatment percent necrosis has favorable outcomes compared with historical adult controls.
Keywords: Adults; Doxorubicin; Ewing sarcoma; Ifosfamide; Vincristine.
© AlphaMed Press 2017.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures

References
-
- National Comprehensive Cancer Network. Soft Tissue Sarcomas (1.2015). Available at http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. Accessed September 27, 2015.
-
- Grier HE, Krailo MD, Tarbell NJ et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694–701. - PubMed
-
- Juergens C, Weston C, Lewis I et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO‐E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer 2006;47:22–29. - PubMed
-
- Ladenstein R, Potschger U, Le Deley MC et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro‐EWING 99 trial. J Clin Oncol 2010;8:3284–3291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

