RLR-mediated antiviral innate immunity requires oxidative phosphorylation activity
- PMID: 28710430
- PMCID: PMC5511143
- DOI: 10.1038/s41598-017-05808-w
RLR-mediated antiviral innate immunity requires oxidative phosphorylation activity
Abstract
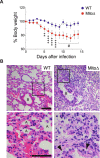
Mitochondria act as a platform for antiviral innate immunity, and the immune system depends on activation of the retinoic acid-inducible gene I (RIG-I)-like receptors (RLR) signaling pathway via an adaptor molecule, mitochondrial antiviral signaling. We report that RLR-mediated antiviral innate immunity requires oxidative phosphorylation (OXPHOS) activity, a prominent physiologic function of mitochondria. Cells lacking mitochondrial DNA or mutant cells with respiratory defects exhibited severely impaired virus-induced induction of interferons and proinflammatory cytokines. Recovery of the OXPHOS activity in these mutants, however, re-established RLR-mediated signal transduction. Using in vivo approaches, we found that mice with OXPHOS defects were highly susceptible to viral infection and exhibited significant lung inflammation. Studies to elucidate the molecular mechanism of OXPHOS-coupled immune activity revealed that optic atrophy 1, a mediator of mitochondrial fusion, contributes to regulate the antiviral immune response. Our findings provide evidence for functional coordination between RLR-mediated antiviral innate immunity and the mitochondrial energy-generating system in mammals.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

