Plasticity in the Oxidative Folding Pathway of the High Affinity Nerita Versicolor Carboxypeptidase Inhibitor (NvCI)
- PMID: 28710462
- PMCID: PMC5511257
- DOI: 10.1038/s41598-017-05657-7
Plasticity in the Oxidative Folding Pathway of the High Affinity Nerita Versicolor Carboxypeptidase Inhibitor (NvCI)
Abstract
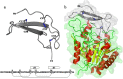
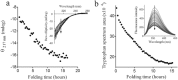
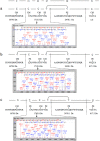
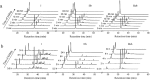
Nerita Versicolor carboxypeptidase inhibitor (NvCI) is the strongest inhibitor reported so far for the M14A subfamily of carboxypeptidases. It comprises 53 residues and a protein fold composed of a two-stranded antiparallel β sheet connected by three loops and stabilized by three disulfide bridges. Here we report the oxidative folding and reductive unfolding pathways of NvCI. Much debate has gone on whether protein conformational folding guides disulfide bond formation or instead they are disulfide bonds that favour the arrangement of local or global structural elements. We show here that for NvCI both possibilities apply. Under physiological conditions, this protein folds trough a funnelled pathway involving a network of kinetically connected native-like intermediates, all sharing the disulfide bond connecting the two β-strands. In contrast, under denaturing conditions, the folding of NvCI is under thermodynamic control and follows a "trial and error" mechanism, in which an initial quasi-stochastic population of intermediates rearrange their disulfide bonds to attain the stable native topology. Despite their striking mechanistic differences, the efficiency of both folding routes is similar. The present study illustrates thus a surprising plasticity in the folding of this extremely stable small disulfide-rich inhibitor and provides the basis for its redesign for biomedical applications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Crystal structures of carboxypeptidase T complexes with transition-state analogs.J Biomol Struct Dyn. 2018 Nov;36(15):3958-3966. doi: 10.1080/07391102.2017.1404932. Epub 2017 Nov 23. J Biomol Struct Dyn. 2018. PMID: 29129130 No abstract available.
-
Crystal structure of novel metallocarboxypeptidase inhibitor from marine mollusk Nerita versicolor in complex with human carboxypeptidase A4.J Biol Chem. 2012 Mar 16;287(12):9250-8. doi: 10.1074/jbc.M111.330100. Epub 2012 Jan 31. J Biol Chem. 2012. PMID: 22294694 Free PMC article.
-
Study of a major intermediate in the oxidative folding of leech carboxypeptidase inhibitor: contribution of the fourth disulfide bond.J Mol Biol. 2005 Sep 30;352(4):961-75. doi: 10.1016/j.jmb.2005.07.065. J Mol Biol. 2005. PMID: 16126224
-
Disulfide bonds and protein folding.Biochemistry. 2000 Apr 18;39(15):4207-16. doi: 10.1021/bi992922o. Biochemistry. 2000. PMID: 10757967 Review.
-
Overview of the regulation of disulfide bond formation in Peptide and protein folding.Curr Protoc Protein Sci. 2014 Apr 1;76:28.6.1-28.6.6. doi: 10.1002/0471140864.ps2806s76. Curr Protoc Protein Sci. 2014. PMID: 24692015 Review.
Cited by
-
Revisiting the Formation of a Native Disulfide Bond: Consequences for Protein Regeneration and Beyond.Molecules. 2020 Nov 16;25(22):5337. doi: 10.3390/molecules25225337. Molecules. 2020. PMID: 33207635 Free PMC article. Review.
-
A Chemical Biology Approach to Probing the Folding Pathways of the Inhibitory Cystine Knot (ICK) Peptide ProTx-II.Front Chem. 2020 Apr 3;8:228. doi: 10.3389/fchem.2020.00228. eCollection 2020. Front Chem. 2020. PMID: 32309273 Free PMC article.
-
Total synthesis of μ-conotoxin lt5d.RSC Adv. 2018 Oct 30;8(64):36579-36583. doi: 10.1039/c8ra03706j. eCollection 2018 Oct 26. RSC Adv. 2018. PMID: 35558937 Free PMC article.
-
Drug delivery targets and strategies to address mast cell diseases.Expert Opin Drug Deliv. 2023 Feb;20(2):205-222. doi: 10.1080/17425247.2023.2166926. Epub 2023 Jan 29. Expert Opin Drug Deliv. 2023. PMID: 36629456 Free PMC article. Review.
-
MIA40 circumvents the folding constraints imposed by TRIAP1 function.J Biol Chem. 2025 Mar;301(3):108268. doi: 10.1016/j.jbc.2025.108268. Epub 2025 Feb 3. J Biol Chem. 2025. PMID: 39909379 Free PMC article.
References
-
- Creighton TE. The protein folding problem. Science. 1988;240(267):344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

