Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers
- PMID: 28710747
- PMCID: PMC6160389
- DOI: 10.1007/s13238-017-0440-4
Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers
Abstract
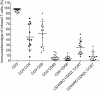
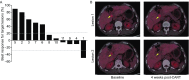
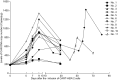
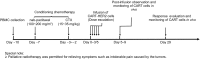
This phase I clinical trial (NCT01935843) is to evaluate the safety, feasibility, and activity of chimeric antigen receptor-engineered T cell (CART) immunotherapy targeting human epidermal growth factor receptor 2 (HER2) in patients with advanced biliary tract cancers (BTCs) and pancreatic cancers (PCs). Eligible patients with HER2-positive (>50%) BTCs and PCs were enrolled in the trial. Well cultured CART-HER2 cells were infused following the conditioning treatment composed of nab-paclitaxel (100-200 mg/m2) and cyclophosphamide (15-35 mg/kg). CAR transgene copy number in the peripheral blood was serially measured to monitor the expansion and persistence of CART-HER2 cells in vivo. Eleven enrolled patients received 1 to 2-cycle CART-HER2 cell infusion (median CAR+ T cell 2.1 × 106/kg). The conditioning treatment resulted in mild-to-moderate fatigue, nausea/vomiting, myalgia/arthralgia, and lymphopenia. Except one grade-3 acute febrile syndrome and one abnormal elevation of transaminase (>9 ULN), adverse events related to the infusion of CART-HER2 cells were mild-to-moderate. Post-infusion toxicities included one case of reversible severe upper gastrointestinal hemorrhage which occurred in a patient with gastric antrum invaded by metastasis 11 days after the CART-HER2 cell infusion, and 2 cases of grade 1-2 delayed fever, accompanied by the release of C-reactive protein and interleukin-6. All patients were evaluable for assessment of clinical response, among which 1 obtained a 4.5-months partial response and 5 achieved stable disease. The median progression free survival was 4.8 months (range, 1.5-8.3 months). Finally, data from this study demonstrated the safety and feasibility of CART-HER2 immunotherapy, and showed encouraging signals of clinical activity.
Keywords: CART; HER2; biliary tract cancers; clinical trial; pancreatic cancers.
Figures

References
-
- Ahmed N, Salsman VS, Kew Y, Shaffer D, Powell S, Zhang YJ, Grossman RG, Heslop HE, Gottschalk S. HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res. 2010;16(2):474–485. doi: 10.1158/1078-0432.CCR-09-1322. - DOI - PMC - PubMed
-
- Ahmed N, Brawley VS, Hegde M, Robertson C, Ghazi A, Gerken C, Liu E, Dakhova O, Ashoori A, Corder A, et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688–1696. doi: 10.1200/JCO.2014.58.0225. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

