Cardiac reprogramming factor Gata4 reduces postinfarct cardiac fibrosis through direct repression of the profibrotic mediator snail
- PMID: 28711329
- PMCID: PMC5647235
- DOI: 10.1016/j.jtcvs.2017.06.035
Cardiac reprogramming factor Gata4 reduces postinfarct cardiac fibrosis through direct repression of the profibrotic mediator snail
Abstract
Objective: The administration of a variety of reprogramming factor cocktails has now been shown to reprogram cardiac fibroblasts into induced cardiomyocyte-like cells. However, reductions in ventricular fibrosis observed after reprogramming factor administration seem to far exceed the extent of induced cardiomyocyte-like cell generation in vivo. We investigated whether reprogramming factor administration might primarily play a role in activating antifibrotic molecular pathways.
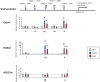
Methods: Adult rat cardiac fibroblasts were infected with lentivirus encoding the transcription factors Gata4, Mef2c, or Tbx5, all 3 vectors, or a green fluorescent protein control vector. Gene and protein expression assays were performed to identify relevant antifibrotic targets of these factors. The antifibrotic effects of these factors were then investigated in a rat coronary ligation model.
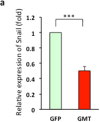
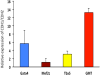
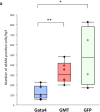
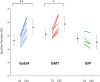
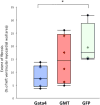
Results: Gata4, Mef2c, or Tbx5 administration to rat cardiac fibroblasts in vitro significantly downregulated expression of Snail and the profibrotic factors connective tissue growth factor, collagen1a1, and fibronectin. Of these factors, Gata4 was shown to be the one responsible for the downregulation of the profibrotic factors and Snail (mRNA expression fold change relative to green fluorescent protein for Snail, Gata4: 0.5 ± 0.3, Mef2c: 1.3 ± 1.0, Tbx5: 0.9 ± 0.5, Gata4, Mef2c, or Tbx5: 0.6 ± 0.2, P < .05). Chromatin immunoprecipitation quantitative polymerase chain reaction identified Gata4 binding sites in the Snail promoter. In a rat coronary ligation model, only Gata4 administration alone improved postinfarct ventricular function and reduced the extent of postinfarct fibrosis.
Conclusions: Gata4 administration reduces postinfarct ventricular fibrosis and improves ventricular function in a rat coronary ligation model, potentially as a result of Gata4-mediated downregulation of the profibrotic mediator Snail.
Keywords: Gata4; Snail; cardiac reprogramming; fibrosis.
Copyright © 2017 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Figures







Comment in
-
More strata and another GATA: Novel roles for GATA4 in postinfarct myocardial repair.J Thorac Cardiovasc Surg. 2017 Nov;154(5):1611-1612. doi: 10.1016/j.jtcvs.2017.07.002. Epub 2017 Jul 18. J Thorac Cardiovasc Surg. 2017. PMID: 28789786 No abstract available.
References
-
- Mathison M, Singh VP, Gersch RP, Ramirez MO, Cooney A, Kaminsky SM, et al. “Triplet” polycistronic vectors encoding Gata4, Mef2c, and Tbx5 enhances postinfarct ventricular functional improvement comared with singlet vectors. J Thorac Cardiovasc Surg. 2014;148:1656–1664.e2. - PubMed
-
- Inagawa K, Miyamoto K, Yamakawa H, Muraoka N, Sadahiro T, Umei T, et al. Induction of cardiomyocyte-like cells in infarct hearts by gene transfer of Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:1147–1156. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

