Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy
- PMID: 28711501
- PMCID: PMC5740213
- DOI: 10.1016/j.bbrc.2017.07.063
Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy
Abstract
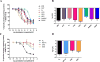
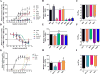
Progestins used in endocrine therapies bind to multiple steroid receptors and are associated with several side-effects. It is thus important to understand the relationship between steroid receptor cross-reactivity and the side-effect profile of progestins. In cell lines that express negligible levels of steroid receptors, we report for the first time the binding affinities, potencies and efficacies of selected progestins from different generations determined in parallel. We show that the progestins bind to the androgen receptor (AR) with similar affinities to each other and progesterone, while none bind estrogen receptor (ER)-β, and only norethisterone acetate, levonorgestrel and gestodene bind ERα. Comparative dose-response analysis revealed that progestins from the first three generations display similar androgenic activity to the natural androgen dihydrotestosterone for transactivation, while norethisterone acetate, levonorgestrel and gestodene are ERα agonists. We show for the first time that the anti-androgenic properties of progesterone and drospirenone are similar to the well-known AR antagonist hydroxyflutamide, while nomegestrol acetate is more potent and nestorone less potent than both hydroxyflutamide and progesterone. Moreover, we are the first to report that the older progestins, unlike progesterone and the fourth generation progestins, are efficacious ERα agonists for transrepression, while the selected progestins from the second and third generation are efficacious AR agonists for transrepression. Considering the progestin potencies and their reported free serum concentrations relative to dihydrotestosterone and estradiol, our results suggest that the progestins are likely to exert AR-, but not ERα- or ERβ-mediated effects in vivo.
Keywords: Androgen receptor; Contraception; Estrogen receptor; Hormone therapy; Progestins.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
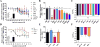
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

