The relevance of contact-independent cell-to-cell transfer of TDP-43 and SOD1 in amyotrophic lateral sclerosis
- PMID: 28711596
- PMCID: PMC6565888
- DOI: 10.1016/j.bbadis.2017.07.007
The relevance of contact-independent cell-to-cell transfer of TDP-43 and SOD1 in amyotrophic lateral sclerosis
Abstract
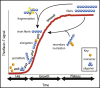
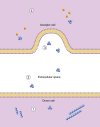
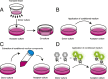
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease involving the formation of cytoplasmic aggregates by proteins including TDP-43 and SOD1, in affected cells in the central nervous system (CNS). Pathology spreads from an initial site of onset to contiguous anatomical regions. There is evidence that for disease-associated proteins, including TDP-43 and SOD1, non-native protein conformers can promote misfolding of the natively folded counterparts, and cell-to-cell transfer of pathological aggregates may underlie the spread of the disease throughout the CNS. A variety of studies have demonstrated that SOD1 is released by neuron-like cells into the surrounding culture medium, either in their free state or encapsulated in extracellular vesicles such as exosomes. Extracellular SOD1 can then be internalised by naïve cells incubated in this conditioned medium, leading to the misfolding and aggregation of endogenous intracellular SOD1; an effect that propagates over serial passages. A similar phenomenon has also been observed with other proteins associated with protein misfolding and progressive neurological disorders, including tau, α-synuclein and both mammalian and yeast prions. Conditioned media experiments using TDP-43 have been less conclusive, with evidence for this protein undergoing intercellular transfer being less straightforward. In this review, we describe the properties of TDP-43 and SOD1 and look at the evidence for their respective abilities to participate in cell-to-cell transfer via conditioned medium, and discuss how variations in the nature of cell-to-cell transfer suggests that a number of different mechanisms are involved in the spreading of pathology in ALS.
Keywords: Aggregates; Conditioned medium; SOD1; Spreading; TDP-43.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Ravits J., Laurie P., Fan Y., Moore D.H. Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007;68:1576–1582. - PubMed
-
- Ravits J., Paul P., Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68:1571–1575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

