Identification of apoB-100 Peptide-Specific CD8+ T Cells in Atherosclerosis
- PMID: 28711866
- PMCID: PMC5586274
- DOI: 10.1161/JAHA.116.005318
Identification of apoB-100 Peptide-Specific CD8+ T Cells in Atherosclerosis
Abstract
Background: T cells are found in atherosclerotic plaques, with evidence supporting a potential role for CD8+ T cells in atherogenesis. Prior studies provide evidence of low-density lipoprotein and apoB-100 reactive T cells, yet specific epitopes relevant to the disease remain to be defined. The current study was undertaken to identify and characterize endogenous, antigen-specific CD8+ T cells in atherosclerosis.
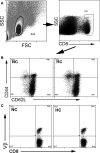
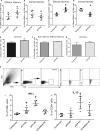
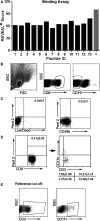
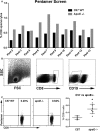
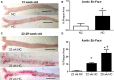
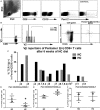
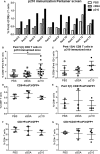
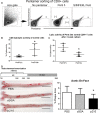
Methods and results: A peptide fragment of apoB-100 that tested positive for binding to the mouse MHC-I allele H2Kb was used to generate a fluorescent-labeled H2Kb pentamer and tested in apoE-/- mice. H2Kb pentamer(+)CD8+ T cells were higher in apoE-/- mice fed an atherogenic diet compared with those fed a normal chow. H2Kb pentamer (+)CD8+ T cells in atherogenic diet-fed mice had significantly increased effector memory phenotype with a shift in Vβ profile. H2Kb pentamer blocked lytic activity of CD8+ T cells from atherogenic diet-fed mice. Immunization of age-matched apoE-/- mice with the apoB-100 peptide altered the immune-dominant epitope of CD8+ T cells and reduced atherosclerosis.
Conclusions: Our study provides evidence of a self-reactive, antigen-specific CD8+ T-cell population in apoE-/- mice. Immune modulation using the peptide antigen reduced atherosclerosis in apoE-/- mice.
Keywords: CD8+ T cells; apoB‐100; atherosclerosis; immunology; lymphocyte; major histocompatibility complex‐I tetramer.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures



References
-
- Dimayuga PC, Chyu KY, Lio WM, Zhao X, Yano J, Zhou J, Honjo T, Shah PK, Cercek B. Reduced neointima formation after arterial injury in CD4‐/‐ mice is mediated by CD8+CD28hi T cells. J Am Heart Assoc. 2013;2:e000155 DOI: 10.1161/JAHA.113.000155. - DOI - PMC - PubMed
-
- Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE‐deficient mice. Circulation. 2013;127:1028–1039. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

