Regulation of Cellular Redox Signaling by Matricellular Proteins in Vascular Biology, Immunology, and Cancer
- PMID: 28712304
- PMCID: PMC5653149
- DOI: 10.1089/ars.2017.7140
Regulation of Cellular Redox Signaling by Matricellular Proteins in Vascular Biology, Immunology, and Cancer
Abstract
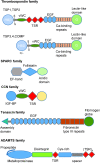
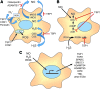
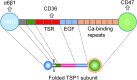
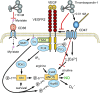
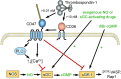
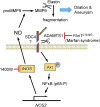
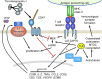
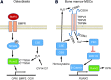
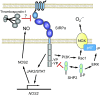
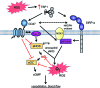
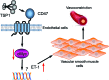
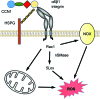
Significance: In contrast to structural elements of the extracellular matrix, matricellular proteins appear transiently during development and injury responses, but their sustained expression can contribute to chronic disease. Through interactions with other matrix components and specific cell surface receptors, matricellular proteins regulate multiple signaling pathways, including those mediated by reactive oxygen and nitrogen species and H2S. Dysregulation of matricellular proteins contributes to the pathogenesis of vascular diseases and cancer. Defining the molecular mechanisms and receptors involved is revealing new therapeutic opportunities. Recent Advances: Thrombospondin-1 (TSP1) regulates NO, H2S, and superoxide production and signaling in several cell types. The TSP1 receptor CD47 plays a central role in inhibition of NO signaling, but other TSP1 receptors also modulate redox signaling. The matricellular protein CCN1 engages some of the same receptors to regulate redox signaling, and ADAMTS1 regulates NO signaling in Marfan syndrome. In addition to mediating matricellular protein signaling, redox signaling is emerging as an important pathway that controls the expression of several matricellular proteins.
Critical issues: Redox signaling remains unexplored for many matricellular proteins. Their interactions with multiple cellular receptors remains an obstacle to defining signaling mechanisms, but improved transgenic models could overcome this barrier.
Future directions: Therapeutics targeting the TSP1 receptor CD47 may have beneficial effects for treating cardiovascular disease and cancer and have recently entered clinical trials. Biomarkers are needed to assess their effects on redox signaling in patients and to evaluate how these contribute to their therapeutic efficacy and potential side effects. Antioxid. Redox Signal. 27, 874-911.
Keywords: CD47; hydrogen sulfide; matricellular proteins; nitric oxide; reactive oxygen species; thrombospondin-1.
Conflict of interest statement
J.S.I. serves as Chair of the Scientific Advisory Board of Radiation Control Technologies, Inc. (RCTI, Garden City, NJ) and has equity interest in RCTI and Tioma Therapeutics (St. Louis, MO) that have licensed CD47 technology for development. The other authors have no competing financial interests to disclose.
Figures
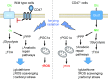

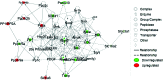
References
-
- Alblas J, Honing H, de Lavalette CR, Brown MH, Dijkstra CD, and van den Berg TK. Signal regulatory protein alpha ligation induces macrophage nitric oxide production through JAK/STAT- and phosphatidylinositol 3-kinase/Rac1/NAPDH oxidase/H2O2-dependent pathways. Mol Cell Biol 25: 7181–7192, 2005 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
