Resident Calmodulin Primes NMDA Receptors for Ca2+-Dependent Inactivation
- PMID: 28712640
- PMCID: PMC5700250
- DOI: 10.1016/j.bpj.2017.06.035
Resident Calmodulin Primes NMDA Receptors for Ca2+-Dependent Inactivation
Abstract
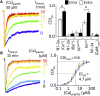
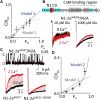
N-methyl-d-aspartate (NMDA) receptors are glutamate- and glycine-gated channels that flux Na+ and Ca2+ into postsynaptic neurons during synaptic transmission. The resulting intracellular Ca2+ transient is essential to physiological and pathological processes related to synaptic development, plasticity, and apoptosis. It also engages calmodulin (CaM) to reduce subsequent NMDA receptor activity in a process known as Ca2+-dependent inactivation (CDI). Here, we used whole-cell electrophysiology to measure CDI and computational modeling to dissect the sequence of events that underlies it. With these approaches, we estimate that CaM senses NMDA receptor Ca2+ influx at ∼9 nm from the channel pore. Further, when we controlled the frequency of Ca2+ influx through individual channels, we found that a kinetic model where apoCaM associates with channels before their activation best predicts the measured CDI. These results provide, to our knowledge, novel functional evidence for CaM preassociation to NMDA receptors in living cells. This particular mechanism for autoinhibitory feedback reveals strategies and challenges for Ca2+ regulation in neurons during physiological synaptic activity and disease.
Published by Elsevier Inc.
Figures





Comment in
-
New Insights into the Mechanism of Ca2+-Dependent Inactivation of NMDA Receptors.Biophys J. 2017 Nov 21;113(10):2131-2132. doi: 10.1016/j.bpj.2017.10.008. Epub 2017 Nov 5. Biophys J. 2017. PMID: 29102038 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

