The Coordinated Action of Calcineurin and Cathepsin D Protects Against α-Synuclein Toxicity
- PMID: 28713240
- PMCID: PMC5491553
- DOI: 10.3389/fnmol.2017.00207
The Coordinated Action of Calcineurin and Cathepsin D Protects Against α-Synuclein Toxicity
Abstract
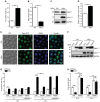
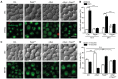
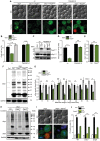
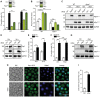
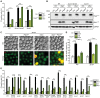
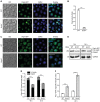
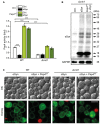
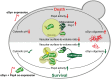
The degeneration of dopaminergic neurons during Parkinson's disease (PD) is intimately linked to malfunction of α-synuclein (αSyn), the main component of the proteinaceous intracellular inclusions characteristic for this pathology. The cytotoxicity of αSyn has been attributed to disturbances in several biological processes conserved from yeast to humans, including Ca2+ homeostasis, general lysosomal function and autophagy. However, the precise sequence of events that eventually results in cell death remains unclear. Here, we establish a connection between the major lysosomal protease cathepsin D (CatD) and the Ca2+/calmodulin-dependent phosphatase calcineurin. In a yeast model for PD, high levels of human αSyn triggered cytosolic acidification and reduced vacuolar hydrolytic capacity, finally leading to cell death. This could be counteracted by overexpression of yeast CatD (Pep4), which re-installed pH homeostasis and vacuolar proteolytic function, decreased αSyn oligomers and aggregates, and provided cytoprotection. Interestingly, these beneficial effects of Pep4 were independent of autophagy. Instead, they required functional calcineurin signaling, since deletion of calcineurin strongly reduced both the proteolytic activity of endogenous Pep4 and the cytoprotective capacity of overexpressed Pep4. Calcineurin contributed to proper endosomal targeting of Pep4 to the vacuole and the recycling of the Pep4 sorting receptor Pep1 from prevacuolar compartments back to the trans-Golgi network. Altogether, we demonstrate that stimulation of this novel calcineurin-Pep4 axis reduces αSyn cytotoxicity.
Keywords: Parkinson’s disease; Pep4; calcineurin; cathepsin D; cytosolic acidification; pH homeostasis; vacuole; α-synuclein.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

