Impaired In Vivo Gamma Oscillations in the Medial Entorhinal Cortex of Knock-in Alzheimer Model
- PMID: 28713250
- PMCID: PMC5491963
- DOI: 10.3389/fnsys.2017.00048
Impaired In Vivo Gamma Oscillations in the Medial Entorhinal Cortex of Knock-in Alzheimer Model
Abstract
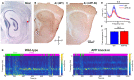
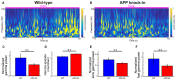
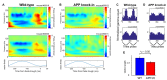
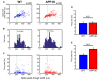
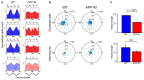
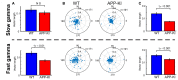
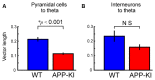
The entorhinal cortex (EC) has bidirectional connections with the hippocampus and plays a critical role in memory formation and retrieval. EC is one of the most vulnerable regions in the brain in early stages of Alzheimer's disease (AD), a neurodegenerative disease with progressive memory impairments. Accumulating evidence from healthy behaving animals indicates gamma oscillations (30-100 Hz) as critical for mediating interactions in the circuit between EC and hippocampus. However, it is still unclear whether gamma oscillations have causal relationship with memory impairment in AD. Here we provide the first evidence that in vivo gamma oscillations in the EC are impaired in an AD mouse model. Cross-frequency coupling of gamma (30-100 Hz) oscillations to theta oscillations was reduced in the medial EC of anesthetized amyloid precursor protein knock-in (APP-KI) mice. Phase locking of spiking activity of layer II/III pyramidal cells to the gamma oscillations was significantly impaired. These data indicate that the neural circuit activities organized by gamma oscillations were disrupted in the medial EC of AD mouse model, and point to gamma oscillations as one of possible mechanisms for cognitive dysfunction in AD patients.
Keywords: Alzheimer’s disease (AD); amyloid precursor protein (APP); gamma oscillations; in vivo electrophysiology; medial entorhinal cortex.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

