A Comparison of Reimbursement Recommendations by European HTA Agencies: Is There Opportunity for Further Alignment?
- PMID: 28713265
- PMCID: PMC5491965
- DOI: 10.3389/fphar.2017.00384
A Comparison of Reimbursement Recommendations by European HTA Agencies: Is There Opportunity for Further Alignment?
Abstract
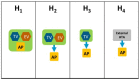
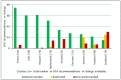
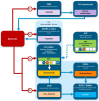
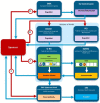
Introduction: In Europe and beyond, the rising costs of healthcare and limited healthcare resources have resulted in the implementation of health technology assessment (HTA) to inform health policy and reimbursement decision-making. European legislation has provided a harmonized route for the regulatory process with the European Medicines Agency, but reimbursement decision-making still remains the responsibility of each country. There is a recognized need to move toward a more objective and collaborative reimbursement environment for new medicines in Europe. Therefore, the aim of this study was to objectively assess and compare the national reimbursement recommendations of 9 European jurisdictions following European Medicines Agency (EMA) recommendation for centralized marketing authorization. Methods: Using publicly available data and newly developed classification tools, this study appraised 9 European reimbursement systems by assessing HTA processes and the relationship between the regulatory, HTA and decision-making organizations. Each national HTA agency was classified according to two novel taxonomies. The System taxonomy, focuses on the position of the HTA agency within the national reimbursement system according to the relationship between the regulator, the HTA-performing agency, and the reimbursement decision-making coverage body. The HTA Process taxonomy distinguishes between the individual HTA agency's approach to economic and therapeutic evaluation and the inclusion of an independent appraisal step. The taxonomic groups were subsequently compared with national HTA recommendations. Results: This study identified European national reimbursement recommendations for 102 new active substances (NASs) approved by the EMA from 2008 to 2012. These reimbursement recommendations were compared using a novel classification tool and identified alignment between the organizational structure of reimbursement systems (System taxonomy) and HTA recommendations. However, there was less alignment between the HTA processes and recommendations. Conclusions: In order to move forward to a more harmonized HTA environment within Europe, it is first necessary to understand the variation in HTA practices within Europe. This study has identified alignment between HTA recommendations and the System taxonomy and one of the major implications of this study is that such alignment could support a more collaborative HTA environment in Europe.
Keywords: HTA; archetypes; health technology assessment; new active substances; pharmaceuticals; process taxonomy; reimbursement recommendations; system taxonomy.
Figures




References
-
- Ascroft R. C., Pichler F. (2014). The Case for HTA Cooperation. MedNous. Available online at: https://lillypad.eu/WP/wp-content/uploads/Ascroft-and-Pichler-2014-MedNo... (Accessed March 20, 2015).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

