Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target
- PMID: 28713289
- PMCID: PMC5491678
- DOI: 10.3389/fphys.2017.00460
Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target
Abstract
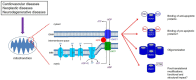
Mitochondria are the key source of ATP that fuels cellular functions, and they are also central in cellular signaling, cell division and apoptosis. Dysfunction of mitochondria has been implicated in a wide range of diseases, including neurodegenerative and cardiac diseases, and various types of cancer. One of the key proteins that regulate mitochondrial function is the voltage-dependent anion channel 1 (VDAC1), the most abundant protein on the outer membrane of mitochondria. VDAC1 is the gatekeeper for the passages of metabolites, nucleotides, and ions; it plays a crucial role in regulating apoptosis due to its interaction with apoptotic and anti-apoptotic proteins, namely members of the Bcl-2 family of proteins and hexokinase. Therefore, regulation of VDAC1 is crucial not only for metabolic functions of mitochondria, but also for cell survival. In fact, multiple lines of evidence have confirmed the involvement of VDAC1 in several diseases. Consequently, modulation or dysregulation of VDAC1 function can potentially attenuate or exacerbate pathophysiological conditions. Understanding the role of VDAC1 in health and disease could lead to selective protection of cells in different tissues and diverse diseases. The purpose of this review is to discuss the role of VDAC1 in the pathogenesis of diseases and as a potentially effective target for therapeutic management of various pathologies.
Keywords: Alzheimer's disease; cardiac ischemia/reperfusion; hexokinase; mitochondria; molecular dynamics; neoplastic diseases; post-translational modification; voltage dependent anion channel.
Figures
References
-
- Alavian K. N., Beutner G., Lazrove E., Sacchetti S., Park H. A., Licznerski P., et al. (2014). An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. U.S.A. 111, 10580–10585. 10.1073/pnas.1401591111 - DOI - PMC - PubMed
-
- Aldakkak M., Camara A. K., Heisner J. S., Yang M., Stowe D. F. (2011). Ranolazine reduces Ca2+ overload and oxidative stress and improves mitochondrial integrity to protect against ischemia reperfusion injury in isolated hearts. Pharmacol. Res. 64, 381–392. 10.1016/j.phrs.2011.06.018 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

