The SpTransformer Gene Family (Formerly Sp185/333) in the Purple Sea Urchin and the Functional Diversity of the Anti-Pathogen rSpTransformer-E1 Protein
- PMID: 28713368
- PMCID: PMC5491942
- DOI: 10.3389/fimmu.2017.00725
The SpTransformer Gene Family (Formerly Sp185/333) in the Purple Sea Urchin and the Functional Diversity of the Anti-Pathogen rSpTransformer-E1 Protein
Abstract
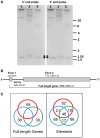
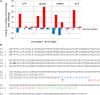
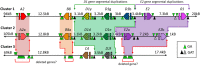
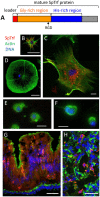
The complex innate immune system of sea urchins is underpinned by several multigene families including the SpTransformer family (SpTrf; formerly Sp185/333) with estimates of ~50 members, although the family size is likely variable among individuals of Strongylocentrotus purpuratus. The genes are small with similar structure, are tightly clustered, and have several types of repeats in the second of two exons and that surround each gene. The density of repeats suggests that the genes are positioned within regions of genomic instability, which may be required to drive sequence diversification. The second exon encodes the mature protein and is composed of blocks of sequence called elements that are present in mosaics of defined element patterns and are the major source of sequence diversity. The SpTrf genes respond swiftly to immune challenge, but only a single gene is expressed per phagocyte. Many of the mRNAs appear to be edited and encode proteins with altered and/or missense sequence that are often truncated, of which some may be functional. The standard SpTrf protein structure is an N-terminal glycine-rich region, a central RGD motif, a histidine-rich region, and a C-terminal region. Function is predicted from a recombinant protein, rSpTransformer-E1 (rSpTrf-E1), which binds to Vibrio and Saccharomyces, but not to Bacillus, and binds tightly to lipopolysaccharide, β-1,3-glucan, and flagellin, but not to peptidoglycan. rSpTrf-E1 is intrinsically disordered but transforms to α helical structure in the presence of binding targets including lipopolysaccharide, which may underpin the characteristics of binding to multiple targets. SpTrf proteins associate with coelomocyte membranes, and rSpTrf-E1 binds specifically to phosphatidic acid (PA). When rSpTrf-E1 is bound to PA in liposome membranes, it induces morphological changes in liposomes that correlate with PA clustering and leakage of luminal contents, and it extracts or removes PA from the bilayer. The multitasking activities of rSpTrf-E1 infer multiple and perhaps overlapping activities for the hundreds of native SpTrf proteins that are produced by individual sea urchins. This likely generates a flexible and highly protective immune system for the sea urchin in its marine habitat that it shares with broad arrays of microbes that may be pathogens and opportunists.
Keywords: Sp185/333; Strongylocentrotus; anti-pathogen; echinoderm; intrinsically disordered proteins; invertebrate; multitasking; purple sea urchin.
Figures



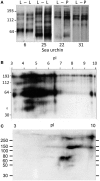
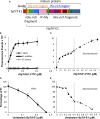
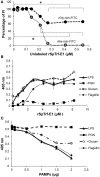
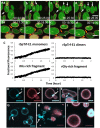


References
-
- Smith LC, Coscia MR. Tuning the host-pathogen relationship through evolution with a special focus on the echinoid Sp185/333 system. Invertebr Survival J (2016) 13:355–73.
-
- Oren M, Barela Hudgell MA, Golconda P, Lun CM, Smith LC. Genomic instability and shared mechanisms for gene diversification in two distant immune gene families: the echinoid 185/333 and the plant NBS-LRR. In: Malagoli D, editor. The Evolution of the Immune System: Conservation and Diversification. London, UK: Elsevier-Academic Press; (2016). p. 295–310.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

