Removal of Acid Orange 7 dye from aqueous solutions by adsorption onto Kenya tea pulps; granulated shape
- PMID: 28713501
- PMCID: PMC5498694
- DOI: 10.19082/4312
Removal of Acid Orange 7 dye from aqueous solutions by adsorption onto Kenya tea pulps; granulated shape
Abstract
Background and aim: Water resources pollution control is one of the main challenges of our time for researchers. Colored wastewater discharges caused by textile industry activities has added to the concern. In this study, removal of Acid Orange 7 dye (AO7) using Kenya Tea residue absorbent (granular) has been studied.
Methods: This cross-sectional study was conducted in 2016. In this work, initially, tea residue was prepared in three forms of raw, treated with concentrated phosphoric acid, and carbonated, at temperatures of 350, 450 and 500 °C in the chemistry laboratory of Gonabad University of Medical Sciences. Then, efficiency of the above absorbents in the removal of Acid Orange 7 dye in initial concentrations of dye as 50-500 mg/l from water samples in terms of pH 2-10 and 1-10 g/l of adsorbent dose within 20 to 300 minutes was investigated. In addition, their subordination from Langmuir and Freundlich absorption isotherms was also determined. Concentration changes in Acid Orange 7 dye at a wavelength of 483 nm was determined by spectrophotometry and results were reported using descriptive statistics.
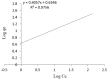
Results: Results showed that efficiency of Acid Orange 7 dye removal is higher in acidic pH and higher adsorbent dosage. The highest efficiency of Acid Orange 7 dye removal was 98.41% by raw tea residue absorbent at pH 2, reaction time was 120 minutes and initial concentration of dye was 50 mg/l, which was obtained at adsorbent dosage of 10 g/l. It was determined that the mechanism of absorption acceptably follows Freundlich absorption isotherm (R2=0.97).
Conclusion: Due to the availability and very low price, optimal performance of Kenya tea raw residue (granular) in Acid Orange 7 dye removal, it can be used as an efficient surface absorber in an absorber from colored wastewater.
Keywords: Acid Orange 7; Adsorption; Aqueous Solutions; Dye Removal; Granulated Shape; Kenya Tea Pulps.
Conflict of interest statement
Conflict of Interest: There is no conflict of interest to be declared.
Figures






References
-
- Yarmohammadi H, Ziaei M, Poursadeghiyan M, Moradi M, Fathi B, Biglari H, et al. Evaluation of Occupational Risk Assessment of Manual Load Carrying Using KIM Method on Auto Mechanics in Kermanshah City in 2015. Research Journal of Medical Sciences. 2016;10(3):116–9. doi: 10.3923/rjmsci.2016.116.119. - DOI
-
- Rahmanpour Salmani E, Ghorbanian A, Ahmadzadeh S, Dolatabadi M, Nemanifar N. Removal of Reactive Red 141 Dye from Synthetic Wastewater by Electrocoagulation Process: Investigation of Operational Parameters. Iranian Journal of Health, Safety and Environment. 2016;3(1):403–11.
-
- Biglari H, Afsharnia M, Alipour V, Khosravi R, Sharafi K, Mahvi AH. A review and investigation of the effect of nanophotocatalytic ozonation process for phenolic compound removal from real effluent of pulp and paper industry. Environ Sci Pollut Res Int. 2017;24(4):4105–16. doi: 10.1007/s11356-016-8079-x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
