Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus
- PMID: 28713774
- PMCID: PMC5491559
- DOI: 10.3389/fcimb.2017.00286
Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus
Abstract
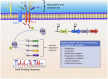
Neutrophils are the most abundant leukocytes in human blood and the first line of defense after bacteria have breached the epithelial barriers. After migration to a site of infection, neutrophils engage and expose invading microorganisms to antimicrobial peptides and proteins, as well as reactive oxygen species, as part of their bactericidal arsenal. Ideally, neutrophils ingest bacteria to prevent damage to surrounding cells and tissues, kill invading microorganisms with antimicrobial mechanisms, undergo programmed cell death to minimize inflammation, and are cleared away by macrophages. Staphylococcus aureus (S. aureus) is a prevalent Gram-positive bacterium that is a common commensal and causes a wide range of diseases from skin infections to endocarditis. Since its discovery, S. aureus has been a formidable neutrophil foe that has challenged the efficacy of this professional assassin. Indeed, proper clearance of S. aureus by neutrophils is essential to positive infection outcome, and S. aureus has developed mechanisms to evade neutrophil killing. Herein, we will review mechanisms used by S. aureus to modulate and evade neutrophil bactericidal mechanisms including priming, activation, chemotaxis, production of reactive oxygen species, and resolution of infection. We will also highlight how S. aureus uses sensory/regulatory systems to tailor production of virulence factors specifically to the triggering signal, e.g., neutrophils and defensins. To conclude, we will provide an overview of therapeutic approaches that may potentially enhance neutrophil antimicrobial functions.
Keywords: Staphylococcus aureus; chemotaxis; host defense; host-pathogen interactions; immune evasion; innate immunity; phagocytosis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

