MUS81 nuclease activity is essential for replication stress tolerance and chromosome segregation in BRCA2-deficient cells
- PMID: 28714477
- PMCID: PMC5520020
- DOI: 10.1038/ncomms15983
MUS81 nuclease activity is essential for replication stress tolerance and chromosome segregation in BRCA2-deficient cells
Erratum in
-
Corrigendum: MUS81 nuclease activity is essential for replication stress tolerance and chromosome segregation in BRCA2-deficient cells.Nat Commun. 2017 Oct 26;8:16171. doi: 10.1038/ncomms16171. Nat Commun. 2017. PMID: 29072253 Free PMC article.
Abstract
Failure to restart replication forks stalled at genomic regions that are difficult to replicate or contain endogenous DNA lesions is a hallmark of BRCA2 deficiency. The nucleolytic activity of MUS81 endonuclease is required for replication fork restart under replication stress elicited by exogenous treatments. Here we investigate whether MUS81 could similarly facilitate DNA replication in the context of BRCA2 abrogation. Our results demonstrate that replication fork progression in BRCA2-deficient cells requires MUS81. Failure to complete genome replication and defective checkpoint surveillance enables BRCA2-deficient cells to progress through mitosis with under-replicated DNA, which elicits severe chromosome interlinking in anaphase. MUS81 nucleolytic activity is required to activate compensatory DNA synthesis during mitosis and to resolve mitotic interlinks, thus facilitating chromosome segregation. We propose that MUS81 provides a mechanism of replication stress tolerance, which sustains survival of BRCA2-deficient cells and can be exploited therapeutically through development of specific inhibitors of MUS81 nuclease activity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
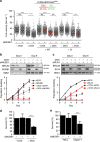
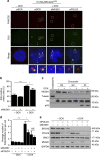
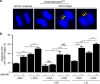

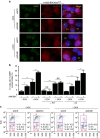
References
-
- Negrini S., Gorgoulis V. G. & Halazonetis T. D. Genomic instability-an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11, 220–228 (2010). - PubMed
-
- Choi E. et al.. BRCA2 fine-tunes the spindle assembly checkpoint through reinforcement of BubR1 acetylation. Dev. Cell 22, 295–308 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

