Acetylcholine contributes to the integration of self-movement cues in head direction cells
- PMID: 28714717
- PMCID: PMC5535758
- DOI: 10.1037/bne0000205
Acetylcholine contributes to the integration of self-movement cues in head direction cells
Abstract
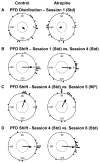
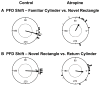
Acetylcholine contributes to accurate performance on some navigational tasks, but details of its contribution to the underlying brain signals are not fully understood. The medial septal area provides widespread cholinergic input to various brain regions, but selective damage to medial septal cholinergic neurons generally has little effect on landmark-based navigation, or the underlying neural representations of location and directional heading in visual environments. In contrast, the loss of medial septal cholinergic neurons disrupts navigation based on path integration, but no studies have tested whether these path integration deficits are associated with disrupted head direction (HD) cell activity. Therefore, we evaluated HD cell responses to visual cue rotations in a familiar arena, and during navigation between familiar and novel arenas, after muscarinic receptor blockade with systemic atropine. Atropine treatment reduced the peak firing rate of HD cells, but failed to significantly affect other HD cell firing properties. Atropine also failed to significantly disrupt the dominant landmark control of the HD signal, even though we used a procedure that challenged this landmark control. In contrast, atropine disrupted HD cell stability during navigation between familiar and novel arenas, where path integration normally maintains a consistent HD cell signal across arenas. These results suggest that acetylcholine contributes to path integration, in part, by facilitating the use of idiothetic cues to maintain a consistent representation of directional heading. (PsycINFO Database Record
(c) 2017 APA, all rights reserved).
Figures

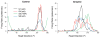

References
-
- Baxter MG, Bucci DJ, Sobel TJ, Williams MJ, Gorman LK, Gallagher M. Intact spatial learning following lesions of basal forebrain cholinergic neurons. Neuroreport. 1996;7:1417–1420. - PubMed
-
- Baxter MG, Gallagher M. Intact spatial learning in both young and aged rats following selective removal of hippocampal cholinergic input. Behav Neurosci. 1996a;110:460–467. - PubMed
-
- Baxter MG, Gallagher M. Neurobiological substrates of behavioral decline: models and data analytic strategies for individual differences in aging. Neurobiol Aging. 1996b;17:491–495. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

