Microglia in prion diseases
- PMID: 28714865
- PMCID: PMC5669569
- DOI: 10.1172/JCI90605
Microglia in prion diseases
Abstract
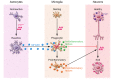
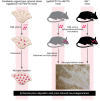
Prion diseases are a group of progressive and fatal neurodegenerative disorders characterized by deposition of scrapie prion protein (PrPSc) in the CNS. This deposition is accompanied by neuronal loss, spongiform change, astrogliosis, and conspicuous microglial activation. Here, we argue that microglia play an overall neuroprotective role in prion pathogenesis. Several microglia-related molecules, such as Toll-like receptors (TLRs), the complement system, cytokines, chemokines, inflammatory regulators, and phagocytosis mediators, are involved in prion pathogenesis. However, the molecular mechanisms underlying the microglial response to prion infection are largely unknown. Consequently, we lack a comprehensive understanding of the regulatory network of microglial activation. On the positive side, recent findings suggest that therapeutic strategies modulating microglial activation and function may have merit in prion disease. Moreover, studies on the role of microglia in prion disease could deepen our understanding of neuroinflammation in a broad range of neurodegenerative disorders.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

