Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency
- PMID: 28714868
- PMCID: PMC5531421
- DOI: 10.1172/JCI92684
Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency
Abstract
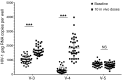
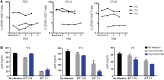
Background: The histone deacetylase (HDAC) inhibitor vorinostat (VOR) can increase HIV RNA expression in vivo within resting CD4+ T cells of aviremic HIV+ individuals. However, while studies of VOR or other HDAC inhibitors have reported reversal of latency, none has demonstrated clearance of latent infection. We sought to identify the optimal dosing of VOR for effective serial reversal of HIV latency.
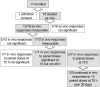
Methods: In a study of 16 HIV-infected, aviremic individuals, we measured resting CD4+ T cell-associated HIV RNA ex vivo and in vivo following a single exposure to VOR, and then in vivo after a pair of doses separated by 48 or 72 hours, and finally following a series of 10 doses given at 72-hour intervals.
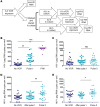
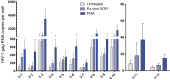
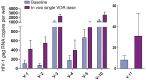
Results: Serial VOR exposures separated by 72 hours most often resulted in an increase in cell-associated HIV RNA within circulating resting CD4+ T cells. VOR was well tolerated by all participants. However, despite serial reversal of latency over 1 month of VOR dosing, we did not observe a measurable decrease (>0.3 log10) in the frequency of latent infection within resting CD4+ T cells.
Conclusions: These findings outline parameters for the experimental use of VOR to clear latent infection. Latency reversal can be achieved by VOR safely and repeatedly, but effective depletion of persistent HIV infection will require additional advances. In addition to improvements in latency reversal, these advances may include the sustained induction of potent antiviral immune responses capable of recognizing and clearing the rare cells in which HIV latency has been reversed.
Trial registration: Clinicaltrials.gov NCT01319383.
Funding: NIH grants U01 AI095052, AI50410, and P30 CA016086 and National Center for Advancing Translational Sciences grant KL2 TR001109.
Conflict of interest statement
Figures

Similar articles
-
Cellular Gene Modulation of HIV-Infected CD4 T Cells in Response to Serial Treatment with the Histone Deacetylase Inhibitor Vorinostat.J Virol. 2020 Jun 16;94(13):e00351-20. doi: 10.1128/JVI.00351-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32295913 Free PMC article.
-
HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat.J Infect Dis. 2014 Sep 1;210(5):728-35. doi: 10.1093/infdis/jiu155. Epub 2014 Mar 11. J Infect Dis. 2014. PMID: 24620025 Free PMC article.
-
Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing.PLoS Pathog. 2014 Apr 10;10(4):e1004071. doi: 10.1371/journal.ppat.1004071. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24722454 Free PMC article. Clinical Trial.
-
CXCR4 Targeting Nanoplatform for Transcriptional Activation of Latent HIV-1 Infected T Cells.ACS Appl Bio Mater. 2024 Aug 19;7(8):4831-4842. doi: 10.1021/acsabm.3c00456. Epub 2023 Aug 16. ACS Appl Bio Mater. 2024. PMID: 37586084 Review.
-
HDAC inhibitors in HIV.Immunol Cell Biol. 2012 Jan;90(1):47-54. doi: 10.1038/icb.2011.95. Epub 2011 Nov 15. Immunol Cell Biol. 2012. PMID: 22083528 Review.
Cited by
-
The BAF complex inhibitor pyrimethamine reverses HIV-1 latency in people with HIV-1 on antiretroviral therapy.Sci Adv. 2023 Mar 17;9(11):eade6675. doi: 10.1126/sciadv.ade6675. Epub 2023 Mar 15. Sci Adv. 2023. PMID: 36921041 Free PMC article. Clinical Trial.
-
New Concepts in Therapeutic Manipulation of HIV-1 Transcription and Latency: Latency Reversal versus Latency Prevention.Viruses. 2023 Jul 31;15(8):1677. doi: 10.3390/v15081677. Viruses. 2023. PMID: 37632019 Free PMC article. Review.
-
Identification of isoform-selective hydroxamic acid derivatives that potently reactivate HIV from latency.J Virus Erad. 2019 Apr 1;5(2):84-91. doi: 10.1016/S2055-6640(20)30057-1. J Virus Erad. 2019. PMID: 31191911 Free PMC article.
-
Highlights from the 8th International Workshop on HIV Persistence during Therapy, 12-15 December 2017, Miami, FL, USA.J Virus Erad. 2018 Apr 1;4(2):132-142. doi: 10.1016/S2055-6640(20)30258-2. J Virus Erad. 2018. PMID: 29682308 Free PMC article.
-
Potent latency reversal by Tat RNA-containing nanoparticle enables multi-omic analysis of the HIV-1 reservoir.Nat Commun. 2023 Dec 18;14(1):8397. doi: 10.1038/s41467-023-44020-5. Nat Commun. 2023. PMID: 38110433 Free PMC article.
References
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

