Antiviral Lipopeptide-Cell Membrane Interaction Is Influenced by PEG Linker Length
- PMID: 28714870
- PMCID: PMC5776016
- DOI: 10.3390/molecules22071190
Antiviral Lipopeptide-Cell Membrane Interaction Is Influenced by PEG Linker Length
Abstract
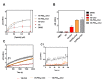
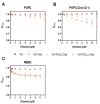
A set of lipopeptides was recently reported for their broad-spectrum antiviral activity against viruses belonging to the Paramyxoviridae family, including human parainfluenza virus type 3 and Nipah virus. Among them, the peptide with a 24-unit PEG linker connecting it to a cholesterol moiety (VG-PEG24-Chol) was found to be the best membrane fusion inhibitory peptide. Here, we evaluated the interaction of the same set of peptides with biomembrane model systems and isolated human peripheral blood mononuclear cells (PBMC). VG-PEG24-Chol showed the highest insertion rate and it was among the peptides that induced a larger change on the surface pressure of cholesterol rich membranes. This peptide also displayed a high affinity towards PBMC membranes. These data provide new information about the dynamics of peptide-membrane interactions of a specific group of antiviral peptides, known for their potential as multipotent paramyxovirus antivirals.
Keywords: antiviral; cholesterol; membranes; paramyxoviruses; peptides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Englund J.A., Moscona A. Paramyxoviruses: Parainfluenza Viruses. In: Kaslow R.A., Stanberry L.R., Le Duc J.W., editors. Viral Infections of Humans. Springer US; New York, NY, USA: 2014. pp. 579–600.
-
- St Vincent M.R., Colpitts C.C., Ustinov A.V, Muqadas M., Joyce M.A., Barsby N.L., Epand R.F., Epand R.M., Khramyshev S.A., Valueva O.A., et al. Rigid amphipathic fusion inhibitors, small molecule antiviral compounds against enveloped viruses. Proc. Natl. Acad. USA. 2010;107:17339–17344. doi: 10.1073/pnas.1010026107. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

