Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing
- PMID: 28714933
- PMCID: PMC5536033
- DOI: 10.3390/ijms18071545
Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing
Abstract
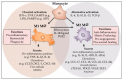
Macrophages and inflammation play a beneficial role during wound repair with macrophages regulating a wide range of processes, such as removal of dead cells, debris and pathogens, through to extracellular matrix deposition re-vascularisation and wound re-epithelialisation. To perform this range of functions, these cells develop distinct phenotypes over the course of wound healing. They can present with a pro-inflammatory M1 phenotype, more often found in the early stages of repair, through to anti-inflammatory M2 phenotypes that are pro-repair in the latter stages of wound healing. There is a continuum of phenotypes between these ranges with some cells sharing phenotypes of both M1 and M2 macrophages. One of the less pleasant consequences of quick closure, namely the replacement with scar tissue, is also regulated by macrophages, through their promotion of fibroblast proliferation, myofibroblast differentiation and collagen deposition. Alterations in macrophage number and phenotype disrupt this process and can dictate the level of scar formation. It is also clear that dysregulated inflammation and altered macrophage phenotypes are responsible for hindering closure of chronic wounds. The review will discuss our current knowledge of macrophage phenotype on the repair process and how alterations in the phenotypes might alter wound closure and the final repair quality.
Keywords: chronic venous disease; chronic wound; diabetes; fibrosis; macrophage; monocyte; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kim M.H., Liu W., Borjesson D.L., Curry F.R., Miller L.S., Cheung A.L., Liu F.T., Isseroff R.R., Simon S.I. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J. Investig. Dermatol. 2008;128:1812–1820. doi: 10.1038/sj.jid.5701223. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

