MacroEvoLution: A New Method for the Rapid Generation of Novel Scaffold-Diverse Macrocyclic Libraries
- PMID: 28715083
- PMCID: PMC5601232
- DOI: 10.1002/chem.201703209
MacroEvoLution: A New Method for the Rapid Generation of Novel Scaffold-Diverse Macrocyclic Libraries
Abstract
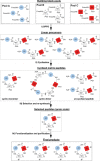
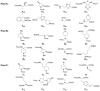
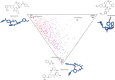
Macrocycles are a structural class bearing great promise for future challenges in medicinal chemistry. Nevertheless, there are few flexible approaches for the rapid generation of structurally diverse macrocyclic compound collections. Here, an efficient method for the generation of novel macrocyclic peptide-based scaffolds is reported. The process, named here as "MacroEvoLution", is based on a cyclization screening approach that gives reliable access to novel macrocyclic architectures. Classification of building blocks into specific pools ensures that scaffolds with orthogonally addressable functionalities are generated, which can easily be used for the generation of structurally diverse compound libraries. The method grants rapid access to novel scaffolds with scalable synthesis (multi gram scale) and the introduction of further diversity at a late stage. Despite being developed for peptidic systems, the approach can easily be extended for the synthesis of systems with a decreased peptidic character.
Keywords: cyclization; diversity oriented synthesis; macrocycles; scaffold diversity; solid-phase synthesis.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures


References
-
- Giordanetto F., Kihlberg J., J. Med. Chem. 2014, 57, 278–295. - PubMed
-
- Matsson P., Doak B. C., Over B., Kihlberg J., Adv. Drug Delivery Rev. 2016, 101, 42–61. - PubMed
-
- Doak B. C., Zheng J., Dobritzsch D., Kihlberg J., J. Med. Chem. 2016, 59, 2312–2327. - PubMed
-
- Driggers E. M., Hale S. P., Lee J., Terrett N. K., Nat. Rev. Drug Discovery 2008, 7, 608–624. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

