Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib
- PMID: 28715249
- PMCID: PMC5590803
- DOI: 10.1200/JCO.2017.72.8519
Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib
Abstract
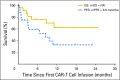
Purpose We evaluated the safety and feasibility of anti-CD19 chimeric antigen receptor-modified T (CAR-T) cell therapy in patients with chronic lymphocytic leukemia (CLL) who had previously received ibrutinib. Methods Twenty-four patients with CLL received lymphodepleting chemotherapy and anti-CD19 CAR-T cells at one of three dose levels (2 × 105, 2 × 106, or 2 × 107 CAR-T cells/kg). Nineteen patients experienced disease progression while receiving ibrutinib, three were ibrutinib intolerant, and two did not experience progression while receiving ibrutinib. Six patients were venetoclax refractory, and 23 had a complex karyotype and/or 17p deletion. Results Four weeks after CAR-T cell infusion, the overall response rate (complete response [CR] and/or partial response [PR]) by International Workshop on Chronic Lymphocytic Leukemia (IWCLL) criteria was 71% (17 of 24). Twenty patients (83%) developed cytokine release syndrome, and eight (33%) developed neurotoxicity, which was reversible in all but one patient with a fatal outcome. Twenty of 24 patients received cyclophosphamide and fludarabine lymphodepletion and CD19 CAR-T cells at or below the maximum tolerated dose (≤ 2 × 106 CAR-T cells/kg). In 19 of these patients who were restaged, the overall response rate by IWCLL imaging criteria 4 weeks after infusion was 74% (CR, 4/19, 21%; PR, 10/19, 53%), and 15/17 patients (88%) with marrow disease before CAR-T cells had no disease by flow cytometry after CAR-T cells. Twelve of these patients underwent deep IGH sequencing, and seven (58%) had no malignant IGH sequences detected in marrow. Absence of the malignant IGH clone in marrow of patients with CLL who responded by IWCLL criteria was associated with 100% progression-free survival and overall survival (median 6.6 months follow-up) after CAR-T cell immunotherapy. The progression-free survival was similar in patients with lymph node PR or CR by IWCLL criteria. Conclusion CD19 CAR-T cells are highly effective in high-risk patients with CLL after they experience treatment failure with ibrutinib therapy.
Figures




References
-
- Döhner H, Stilgenbauer S, Benner A, et al. : Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 343:1910-1916, 2000 - PubMed
-
- Stilgenbauer S, Schnaiter A, Paschka P, et al. : Gene mutations and treatment outcome in chronic lymphocytic leukemia: Results from the CLL8 trial. Blood 123:3247-3254, 2014 - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. : Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 376:1164-1174, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

